ATcT [1 , 2 ] enthalpies of formation based on version 1.122r of the Thermochemical Network [3 ] version 1.122q [4, 5 ] to include a non-rigid rotor anharmonic oscillator (NRRAO) partition function for hydroxymethyl [6 ], as well as data on 42 additional species, some of which are related to soot formation mechanisms.
Species Name acts as a link to the page
itemizing the most important contributors to the provenance of its ATcT enthalpy of formation, other highly correlated chemical species, and high-leverage reactions in the Thermochemical Network.3 ], and, if possible, the ATcT approach as given in Refs. [1 ] and [2 ].
Reaction Search
The stoichiometry represents fractions as decimals,
Species Name Preferred Formula Image Δf H°(0 K)
Δf H°(298.15 K) Uncertainty Units Relative ATcT ID
Dihydrogen H2 (g) 0 0 exact 2.01588 ± 1333-74-0*0 Helium He (g) 0 0 exact 4.0026020 ± 7440-59-7*0 Carbon C (graphite) 0 0 exact 12.01070 ± 7440-44-0*500 Dinitrogen N2 (g) 0 0 exact 28.01348 ± 7727-37-9*0 Dioxygen O2 (g) 0 0 exact 31.99880 ± 7782-44-7*0 Difluorine F2 (g) 0 0 exact 37.9968064 ± 7782-41-4*0 Neon Ne (g) 0 0 exact 20.17970 ± 7440-01-9*0 Silicon Si (cr,l) 0 0 exact 28.08550 ± 7440-21-3*500 Dichlorine Cl2 (g) 0 0 exact 70.9054 ± 7782-50-5*0 Argon Ar (g) 0 0 exact 39.94800 ± 7440-37-1*0 Dibromine Br2 (cr,l) 0 0 exact 159.8080 ± 7726-95-6*500 Krypton Kr (g) 0 0 exact 83.8000 ± 7439-90-9*0 Diiodine I2 (cr,l) 0 0 exact 253.808940 ± 7553-56-2*500 Xenon Xe (g) 0 0 exact 131.2900 ± 7440-63-3*0 Deuterium molecule D2 (g) 0 0 exact 4.02820355600 ± 7782-39-0*0 Hydrogen atom H (g) 216.034 217.998 ± 0.000 kJ/mol 1.007940 ± 12385-13-6*0 Carbon C (g) 711.398 716.883 ± 0.045 kJ/mol 12.01070 ± 7440-44-0*0 Oxygen atom O (g) 246.844 249.229 ± 0.0021 kJ/mol 15.99940 ± 17778-80-2*0 Nitrogen atom N (g) 470.579 472.442 ± 0.024 kJ/mol 14.006740 ± 17778-88-0*0 Methane CH4 (g) -66.557 -74.526 ± 0.049 kJ/mol 16.04246 ± 74-82-8*0 Carbon dioxide CO2 (g) -393.110 -393.476 ± 0.015 kJ/mol 44.00950 ± 124-38-9*0 Ethane CH3CH3 (g) -68.41 -84.04 ± 0.13 kJ/mol 30.0690 ± 74-84-0*0 Chlorine atom Cl (g) 119.621 121.302 ± 0.0011 kJ/mol 35.45270 ± 22537-15-1*0 Water H2O (cr,l) -286.298 -285.826 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*500 Hydron H+ (g) 1528.084 1530.047 ± 0.000 kJ/mol 1.007391 ± 12408-02-5*0 Water H2O (g) -238.929 -241.832 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*0 Fluorine atom F (g) 77.258 79.364 ± 0.047 kJ/mol 18.99840320 ± 14762-94-8*0 Ethylene CH2CH2 (g) 60.84 52.32 ± 0.12 kJ/mol 28.0532 ± 74-85-1*0 Methyl CH3 (g) 149.866 146.452 ± 0.055 kJ/mol 15.03452 ± 2229-07-4*0 Methanol CH3OH (g) -190.03 -200.91 ± 0.15 kJ/mol 32.04186 ± 67-56-1*0 Bromine atom Br (g) 117.917 111.855 ± 0.056 kJ/mol 79.90400 ± 10097-32-2*0 Propane CH3CH2CH3 (g) -82.78 -105.07 ± 0.16 kJ/mol 44.0956 ± 74-98-6*0 Hydroxyl OH (g) 37.252 37.492 ± 0.026 kJ/mol 17.00734 ± 3352-57-6*0 Hydrogen chloride HCl (g) -91.989 -92.173 ± 0.0062 kJ/mol 36.46064 ± 7647-01-0*0 Carbon monoxide CO (g) -113.804 -110.524 ± 0.026 kJ/mol 28.01010 ± 630-08-0*0 Formaldehyde CH2O (g) -105.376 -109.214 ± 0.096 kJ/mol 30.02598 ± 50-00-0*0 Ethanol CH3CH2OH (g) -217.32 -235.04 ± 0.22 kJ/mol 46.0684 ± 64-17-5*0 Ammonia NH3 (g) -38.565 -45.558 ± 0.029 kJ/mol 17.03056 ± 7664-41-7*0 Acetylene HCCH (g) 228.84 228.28 ± 0.13 kJ/mol 26.0373 ± 74-86-2*0 Hydrogen fluoride HF (g) -272.674 -272.721 ± 0.048 kJ/mol 20.006343 ± 7664-39-3*0 Hydrogen bromide HBr (g) -27.60 -35.45 ± 0.14 kJ/mol 80.9119 ± 10035-10-6*0 Iodine I (g) 107.157 106.757 ± 0.0021 kJ/mol 126.904470 ± 14362-44-8*0 Ethyl CH3CH2 (g) 131.07 119.99 ± 0.25 kJ/mol 29.0611 ± 2025-56-1*0 Hydrogen cyanide HCN (g) 129.665 129.280 ± 0.089 kJ/mol 27.02538 ± 74-90-8*0 Nitric oxide NO (g) 90.631 91.135 ± 0.066 kJ/mol 30.00614 ± 10102-43-9*0 Chloromethane CH3Cl (g) -74.31 -82.24 ± 0.25 kJ/mol 50.4872 ± 74-87-3*0 Propene CH3CHCH2 (g) 34.89 19.88 ± 0.19 kJ/mol 42.0797 ± 115-07-1*0 Benzene C6H6 (g) 100.63 83.12 ± 0.22 kJ/mol 78.1118 ± 71-43-2*0 Propyne CH3CCH (g) 192.70 185.64 ± 0.22 kJ/mol 40.0639 ± 74-99-7*0 Nitrogen dioxide ONO (g) 36.871 34.064 ± 0.066 kJ/mol 46.00554 ± 10102-44-0*0 Hydrogen iodide HI (g) 28.646 26.471 ± 0.036 kJ/mol 127.912410 ± 10034-85-2*0 Hydroxyde [OH]- (g) -139.089 -139.056 ± 0.026 kJ/mol 17.00789 ± 14280-30-9*0 Dioxidanyl HO2 (g) 15.12 12.21 ± 0.15 kJ/mol 33.00674 ± 3170-83-0*0 Acetaldehyde CH3CHO (g) -155.06 -165.54 ± 0.25 kJ/mol 44.0526 ± 75-07-0*0 Fluoromethane CH3F (g) -227.46 -235.49 ± 0.23 kJ/mol 34.03292 ± 593-53-3*0 Hydrogen peroxide H2O2 (g) -129.472 -135.458 ± 0.063 kJ/mol 34.01468 ± 7722-84-1*0 n-Butane CH3CH2CH2CH3 (g) -98.44 -125.75 ± 0.24 kJ/mol 58.1222 ± 106-97-8*0 Allene CH2CCH2 (g) 197.27 189.78 ± 0.24 kJ/mol 40.0639 ± 463-49-0*0 Bromomethane CH3Br (g) -20.21 -35.61 ± 0.26 kJ/mol 94.9385 ± 74-83-9*0 Silicon Si (g) 450.53 454.86 ± 0.64 kJ/mol 28.08550 ± 7440-21-3*0 Methylamine CH3NH2 (g) -6.52 -21.23 ± 0.24 kJ/mol 31.05714 ± 74-89-5*0 Methylene CH2 (g, singlet) 428.72 429.13 ± 0.11 kJ/mol 14.02658 ± 2465-56-7*2 Methylene CH2 (g, triplet) 391.060 391.617 ± 0.097 kJ/mol 14.02658 ± 2465-56-7*1 Vinyl CH2CH (g) 301.21 297.01 ± 0.31 kJ/mol 27.0452 ± 2669-89-8*0 Formic acid HC(O)OH (g, syn) -371.09 -378.40 ± 0.22 kJ/mol 46.0254 ± 64-18-6*1 Methoxy CH3O (g) 28.80 21.43 ± 0.28 kJ/mol 31.03392 ± 2143-68-2*0 Hypochlorous acid HOCl (g) -73.866 -76.805 ± 0.027 kJ/mol 52.46004 ± 7790-92-3*0 Tetrafluoromethane CF4 (g) -927.50 -933.47 ± 0.25 kJ/mol 88.00431 ± 75-73-0*0 Dibromine Br2 (g) 45.68 30.89 ± 0.12 kJ/mol 159.8080 ± 7726-95-6*0 Acetone CH3C(O)CH3 (g) -200.05 -216.74 ± 0.35 kJ/mol 58.0791 ± 67-64-1*0 Tetrachloromethane CCl4 (g) -93.44 -95.61 ± 0.43 kJ/mol 153.8215 ± 56-23-5*0 Fluoride F- (g) -250.907 -249.122 ± 0.047 kJ/mol 18.99895178 ± 16984-48-8*0 Diiodine I2 (g) 65.497 62.417 ± 0.0041 kJ/mol 253.808940 ± 7553-56-2*0 iso-Butane CH(CH3)3 (g) -106.02 -134.62 ± 0.30 kJ/mol 58.1222 ± 75-28-5*0 1,3-Butadiene CH2CHCHCH2 (g) 125.38 110.90 ± 0.33 kJ/mol 54.0904 ± 106-99-0*0 Ketene CH2CO (g) -45.35 -48.47 ± 0.11 kJ/mol 42.0367 ± 463-51-4*0 Chloroethane CH3CH2Cl (g) -96.90 -111.49 ± 0.26 kJ/mol 64.5138 ± 75-00-3*0 Phenyl C6H5 (g) 350.67 337.31 ± 0.51 kJ/mol 77.1039 ± 2396-01-2*0 Hydroxymethyl CH2OH (g) -10.44 -16.75 ± 0.27 kJ/mol 31.03392 ± 2597-43-5*0 Toluene C6H5CH3 (g) 73.39 50.11 ± 0.33 kJ/mol 92.1384 ± 108-88-3*0 Methylidyne CH (g) 592.825 596.159 ± 0.097 kJ/mol 13.01864 ± 3315-37-5*0 Methoxide [CH3O]- (g) -122.59 -130.32 ± 0.28 kJ/mol 31.03447 ± 3315-60-4*0 Nitrilomethyl CN (g) 436.76 440.04 ± 0.14 kJ/mol 26.01744 ± 2074-87-5*0 Acetonitrile CH3CN (g) 81.11 74.05 ± 0.25 kJ/mol 41.0520 ± 75-05-8*0 Silane SiH4 (g) 42.88 33.27 ± 0.70 kJ/mol 32.11726 ± 7803-62-5*0 Propargyl CH2CCH (g) 353.94 351.35 ± 0.33 kJ/mol 39.0559 ± 2932-78-7*0 Ozone OOO (g) 144.397 141.735 ± 0.039 kJ/mol 47.99820 ± 10028-15-6*0 Acetic acid CH3C(O)OH (g, syn) -418.61 -432.73 ± 0.38 kJ/mol 60.0520 ± 64-19-7*1 Oxonium [H3O]+ (g) 605.85 599.10 ± 0.17 kJ/mol 19.02267 ± 13968-08-6*0 Fluoroform CHF3 (g) -689.05 -696.00 ± 0.41 kJ/mol 70.01385 ± 75-46-7*0 Trifluoromethyl CF3 (g) -464.95 -467.75 ± 0.45 kJ/mol 69.00591 ± 2264-21-3*0 Ethylium [CH3CH2]+ (g) 914.95 902.88 ± 0.30 kJ/mol 29.0606 ± 14936-94-8*0 Phenol C6H5OH (g) -74.74 -93.34 ± 0.63 kJ/mol 94.1112 ± 108-95-2*0 Chloride Cl- (g) -228.953 -227.346 ± 0.0021 kJ/mol 35.45325 ± 16887-00-6*0 Bromide Br- (g) -206.619 -212.682 ± 0.056 kJ/mol 79.90455 ± 24959-67-9*0 Oxomethylium [HCO]+ (g) 827.770 827.185 ± 0.096 kJ/mol 29.01749 ± 17030-74-9*0 Hypobromous acid HOBr (g) -51.51 -61.99 ± 0.47 kJ/mol 96.9113 ± 13517-11-8*0 Amidogen NH2 (g) 188.92 186.03 ± 0.11 kJ/mol 16.02262 ± 13770-40-6*0 Formyl HCO (g) 41.394 41.770 ± 0.096 kJ/mol 29.01804 ± 2597-44-6*0 Chlorooxidanyl ClO (g) 101.124 101.716 ± 0.035 kJ/mol 51.45210 ± 14989-30-1*0 Formamide CH(O)NH2 (g) -178.57 -188.94 ± 0.36 kJ/mol 45.04066 ± 75-12-7*0 Methyl hydroperoxide CH3OOH (g) -114.92 -127.74 ± 0.50 kJ/mol 48.0413 ± 3031-73-0*0 Allyl CH2CHCH2 (g) 180.02 168.30 ± 0.42 kJ/mol 41.0718 ± 1981-80-2*0 Imidogen NH (g) 358.74 358.79 ± 0.16 kJ/mol 15.014680 ± 13774-92-0*0 Dioxymethyl CH2OO (g) 111.95 104.95 ± 0.62 kJ/mol 46.0254 ± 56077-92-0*0 Cyanoacetylene HCCCN (g) 372.10 373.84 ± 0.62 kJ/mol 51.0468 ± 1070-71-9*0 Nitric acid HON(O)O (g) -124.50 -134.21 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*0 Ethoxide [CH3CH2O]- (g) -163.98 -178.92 ± 0.46 kJ/mol 45.0610 ± 16331-64-9*0 Ethenol CH2CHOH (g, syn) -113.11 -124.29 ± 0.52 kJ/mol 44.0526 ± 557-75-5*1 Vinyl chloride CH2CHCl (g) 29.28 21.70 ± 0.30 kJ/mol 62.4979 ± 75-01-4*0 Methylium [CH3]+ (g) 1099.339 1095.396 ± 0.050 kJ/mol 15.03397 ± 14531-53-4*0 Iodide I- (g) -187.995 -188.396 ± 0.0021 kJ/mol 126.905019 ± 20461-54-5*0 Cyanogen chloride ClCN (g) 134.98 135.67 ± 0.46 kJ/mol 61.4701 ± 506-77-4*0 Methylperoxy CH3OO (g) 22.46 13.01 ± 0.38 kJ/mol 47.0333 ± 2143-58-0*0 Hydroxyformyl HOCO (g, trans) -181.14 -184.10 ± 0.44 kJ/mol 45.0174 ± 2564-86-5*1 Isocyanic acid HNCO (g) -115.97 -118.96 ± 0.28 kJ/mol 43.02478 ± 75-13-8*0 Difluorophosgene CF2O (g) -603.34 -606.44 ± 0.41 kJ/mol 66.00691 ± 353-50-4*0 Vinylacetylene CH2CHCCH (g) 296.17 289.41 ± 0.58 kJ/mol 52.0746 ± 689-97-4*0 Hypofluorous acid HOF (g) -84.39 -87.29 ± 0.19 kJ/mol 36.00574 ± 14034-79-8*0 Hydrogen chloride HCl (aq, 600 H2O) -166.452 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*834 Dichloromethane CH2Cl2 (g) -86.52 -93.38 ± 0.52 kJ/mol 84.9320 ± 75-09-2*0 1,3-Butadiyne HCCCCH (g) 458.27 460.00 ± 0.58 kJ/mol 50.0587 ± 460-12-8*0 Chlorobenzene C6H5Cl (g) 67.15 52.20 ± 0.61 kJ/mol 112.5566 ± 108-90-7*0 Ethynyl CCH (g) 563.88 567.99 ± 0.14 kJ/mol 25.0293 ± 2122-48-7*0 Cyanogen bromide BrCN (g) 187.1 180.5 ± 1.1 kJ/mol 105.9214 ± 506-68-3*0 Iodomethane CH3I (g) 24.51 14.98 ± 0.18 kJ/mol 141.93899 ± 74-88-4*0 Oxygen atom anion O- (g) 105.868 108.097 ± 0.0021 kJ/mol 15.99995 ± 14337-01-0*0 n-Propyl CH3CH2CH2 (g) 118.26 100.85 ± 0.51 kJ/mol 43.0877 ± 2143-61-5*0 iso-Propyl CH3CHCH3 (g) 105.30 88.43 ± 0.49 kJ/mol 43.0877 ± 2025-55-0*0 Trihydrogen cation [H3]+ (g) 1110.304 1106.696 ± 0.013 kJ/mol 3.02327 ± 28132-48-1*0 Acetyl CH3CO (g) -3.62 -10.28 ± 0.32 kJ/mol 43.0446 ± 3170-69-2*0 Cyclopropene CH2(CHCH) (g) 292.27 283.54 ± 0.47 kJ/mol 40.0639 ± 2781-85-3*0 Cyclopentadiene CH2(CHCHCHCH) (g) 151.28 134.15 ± 0.63 kJ/mol 66.1011 ± 542-92-7*0 2-Hydroxyethyl CH2CH2OH (g, gauche-syn) -13.37 -26.63 ± 0.56 kJ/mol 45.0605 ± 4422-54-2*1 Tetrabromomethane CBr4 (g) 133.5 103.8 ± 1.3 kJ/mol 331.6267 ± 558-13-4*0 Difluoromethane CH2F2 (g) -443.14 -450.76 ± 0.33 kJ/mol 52.02339 ± 75-10-5*0 Ethynide [CCH]- (g) 277.43 280.84 ± 0.18 kJ/mol 25.0299 ± 29075-95-4*0 Oxygen difluoride FOF (g) 26.82 24.57 ± 0.24 kJ/mol 53.99621 ± 7783-41-7*0 Hypochlorite [ClO]- (g) -118.60 -118.33 ± 0.13 kJ/mol 51.45265 ± 14380-61-1*0 Nitrosyl hydride HNO (g) 109.94 106.97 ± 0.11 kJ/mol 31.01408 ± 14332-28-6*0 2-Butyne CH3CCCH3 (g) 159.43 146.47 ± 0.49 kJ/mol 54.0904 ± 503-17-3*0 Isocyanomethane CH3NC (g) 183.86 177.31 ± 0.49 kJ/mol 41.0520 ± 593-75-9*0 Cyclopropane CH2(CH2CH2) (g) 70.97 53.84 ± 0.40 kJ/mol 42.0797 ± 75-19-4*0 Propynylidene HCCCH (g, triplet) 543.45 546.45 ± 0.65 kJ/mol 38.0480 ± 67152-18-5*1 Butatriene CH2CCCH2 (g) 328.21 321.96 ± 0.65 kJ/mol 52.0746 ± 2873-50-9*0 Methoxymethane CH3OCH3 (g) -166.52 -184.02 ± 0.40 kJ/mol 46.0684 ± 115-10-6*0 Chloroform CHCl3 (g) -97.16 -102.07 ± 0.50 kJ/mol 119.3767 ± 67-66-3*0 Fluoromethylidyne CF (g) 243.14 246.74 ± 0.12 kJ/mol 31.00910 ± 3889-75-6*0 Acrylonitrile CH2CHCN (g, singlet) 193.75 186.99 ± 0.66 kJ/mol 53.0627 ± 107-13-1*1 Silanol SiH3OH (g) -270.9 -282.6 ± 1.3 kJ/mol 48.11666 ± 14475-38-8*0 Oxygen atom cation O+ (g) 1560.786 1562.644 ± 0.0021 kJ/mol 15.99885 ± 14581-93-2*0 Nitrous acid HONO (g, trans) -72.991 -79.134 ± 0.079 kJ/mol 47.01348 ± 7782-77-6*1 1,2-Propadiene-1,3-diylidene CCC (g) 814.65 823.61 ± 0.54 kJ/mol 36.0321 ± 12075-35-3*0 Cyanic fluoride FCN (g) 8.76 9.09 ± 0.69 kJ/mol 45.01584 ± 1495-50-7*0 1-Hydroxyethyl CH3CHOH (g, gauche-anti) -42.79 -56.22 ± 0.57 kJ/mol 45.0605 ± 2348-46-1*1 Ethoxy CH3CH2O (g, X 2A") 1.15 -12.76 ± 0.45 kJ/mol 45.0605 ± 2154-50-9*51 Formate [HC(O)O]- (g) -463.23 -466.88 ± 0.56 kJ/mol 45.0180 ± 71-47-6*0 Methyl formate HC(O)OCH3 (g, syn) -346.88 -359.93 ± 0.84 kJ/mol 60.0520 ± 107-31-3*1 Dimethylamine (CH3)2NH (g) 4.08 -17.55 ± 0.42 kJ/mol 45.0837 ± 124-40-3*0 Tetrafluoroethylene CF2CF2 (g) -670.99 -674.40 ± 0.52 kJ/mol 100.0150 ± 116-14-3*0 Chlorotrifluoromethane CF3Cl (g) -704.54 -709.65 ± 0.57 kJ/mol 104.4586 ± 75-72-9*0 Ethyl bromide CH3CH2Br (g) -41.12 -63.06 ± 0.25 kJ/mol 108.9651 ± 74-96-4*0 Vinyl bromide CH2CHBr (g) 88.70 73.90 ± 0.57 kJ/mol 106.9492 ± 593-60-2*0 Methanimine CH2NH (g) 96.48 88.57 ± 0.40 kJ/mol 29.04126 ± 2053-29-4*0 Methyl nitrite CH3ONO (g, cis) -55.52 -67.30 ± 0.45 kJ/mol 61.0401 ± 624-91-9*2 Chlorine fluoride ClF (g) -55.617 -55.712 ± 0.053 kJ/mol 54.45110 ± 7790-89-8*0 Diazynium [NNH]+ (g) 1042.43 1038.82 ± 0.68 kJ/mol 29.02087 ± 12357-66-3*0 Nitrite [ONO]- (g) -182.62 -185.42 ± 0.46 kJ/mol 46.00609 ± 14797-65-0*0 Hydroxylamine NH2OH (g, trans) -33.12 -43.50 ± 0.45 kJ/mol 33.02996 ± 7803-49-8*1 Hydride H- (g) 143.264 145.228 ± 0.000 kJ/mol 1.008489 ± 12184-88-2*0 n-Butyl CH3CH2CH2CH2 (g) 102.78 80.28 ± 0.71 kJ/mol 57.1143 ± 2492-36-6*0 Methyl acetate CH3C(O)OCH3 (g, syn) -394.22 -413.09 ± 0.60 kJ/mol 74.0785 ± 79-20-9*1 Phosgene CCl2O (g) -217.45 -219.14 ± 0.27 kJ/mol 98.9155 ± 75-44-5*0 Vinyl fluoride CH2CHF (g) -134.61 -142.42 ± 0.45 kJ/mol 46.0436 ± 75-02-5*0 Phenylium [C6H5]+ (g) 1148.55 1135.77 ± 0.85 kJ/mol 77.1034 ± 17333-73-2*0 Fluorooxidanyl FO (g) 110.27 110.90 ± 0.15 kJ/mol 34.99780 ± 12061-70-0*0 Hypoiodous acid HOI (g) -54.2 -58.9 ± 3.1 kJ/mol 143.91181 ± 14332-21-9*0 Methanium [CH5]+ (g) 921.96 911.71 ± 0.44 kJ/mol 17.04985 ± 15135-49-6*0 Oxirane O(CH2CH2) (g) -40.07 -52.60 ± 0.37 kJ/mol 44.0526 ± 75-21-8*0 cis-2-Butene CH3CHCHCH3 (g, cis) 14.23 -7.05 ± 0.40 kJ/mol 56.1063 ± 590-18-1*0 Formic acid HC(O)OH (g, anti) -354.75 -361.85 ± 0.38 kJ/mol 46.0254 ± 64-18-6*2 Cyanooxidanyl NCO (g) 126.93 127.41 ± 0.33 kJ/mol 42.01684 ± 22400-26-6*0 Methanetetraylbisamidogen NCN (g) 451.24 451.64 ± 0.44 kJ/mol 40.02418 ± 2669-76-3*0 Fluoroethane CH3CH2F (g) -257.27 -272.13 ± 0.36 kJ/mol 48.0595 ± 353-36-6*0 Bromotrifluoromethane CF3Br (g) -638.88 -651.00 ± 0.44 kJ/mol 148.9099 ± 75-63-8*0 Phenide [C6H5]- (g) 244.43 231.01 ± 0.38 kJ/mol 77.1044 ± 30922-78-2*0 Fluorobenzene C6H5F (g) -99.08 -114.76 ± 0.89 kJ/mol 96.1023 ± 462-06-6*0 Nitromethane CH3N(O)O (g) -60.81 -74.71 ± 0.46 kJ/mol 61.0401 ± 75-52-5*0 Acrylonitrile CH2CHCN (g) 193.75 186.99 ± 0.66 kJ/mol 53.0627 ± 107-13-1*0 1,2,3-Butatrien-1-yl CH2CCCH (g) 497.34 497.36 ± 0.70 kJ/mol 51.0666 ± 22112-56-7*0 2,4-Hexadiyne CH3CCCCCH3 (g) 380.15 370.13 ± 0.87 kJ/mol 78.1118 ± 2809-69-0*0 Dioxygen cation [O2]+ (g) 1164.585 1165.221 ± 0.0092 kJ/mol 31.99825 ± 12185-07-8*0 Ethynylene C2 (g) 820.33 828.80 ± 0.26 kJ/mol 24.0214 ± 12070-15-4*0 Methylidyne CH (g, quartet) 664.60 667.93 ± 0.54 kJ/mol 13.01864 ± 3315-37-5*2 Ethanide [CH3CH2]- (g) 155.29 144.21 ± 0.87 kJ/mol 29.0616 ± 25013-41-6*0 Vinylium [CH2CH]+ (g) 1119.07 1115.63 ± 0.55 kJ/mol 27.0447 ± 14604-48-9*0 Vinyl anion [CH2CH]- (g) 237.06 232.64 ± 0.73 kJ/mol 27.0458 ± 25012-81-1*0 Aminomethyl CH2NH2 (g) 159.27 149.42 ± 0.37 kJ/mol 30.04920 ± 10507-29-6*0 Cyanide [CN]- (g) 63.960 67.246 ± 0.093 kJ/mol 26.01799 ± 57-12-5*0 Cyanomethyl CH2CN (g) 263.39 260.51 ± 0.68 kJ/mol 40.0440 ± 2932-82-3*0 Peroxyformic acid HC(O)OOH (g, cis) -279.24 -288.59 ± 0.88 kJ/mol 62.0248 ± 107-32-4*1 Propadienylidene CH2CC (g) 554.37 555.52 ± 0.40 kJ/mol 38.0480 ± 60731-10-4*0 Isobutene CH2C(CH3)2 (g) 4.06 -17.01 ± 0.39 kJ/mol 56.1063 ± 115-11-7*0 Cyclopentane CH2(CH2CH2CH2CH2) (g) -43.85 -76.42 ± 0.42 kJ/mol 70.1329 ± 287-92-3*0 Phenolate [C6H5O]- (g) -146.59 -162.06 ± 0.84 kJ/mol 93.1038 ± 3229-70-7*0 Cyclopentadienyl CH(CHCHCHCH) (g) 274.64 261.53 ± 0.92 kJ/mol 65.0932 ± 2143-53-5*0 Carbamoyl NH2CO (g) -7.78 -14.18 ± 0.74 kJ/mol 44.03272 ± 2858-51-7*0 Tetrahedrane CH(CH(CHCH)) (g) 547.7 539.3 ± 1.2 kJ/mol 52.0746 ± 157-39-1*0 Ethylamine CH3CH2NH2 (g) -28.26 -50.03 ± 0.54 kJ/mol 45.0837 ± 75-04-7*0 Chlorine dioxide OClO (g) 101.42 98.95 ± 0.29 kJ/mol 67.4515 ± 10049-04-4*0 Bromooxidanyl BrO (g) 131.16 123.62 ± 0.28 kJ/mol 95.9034 ± 15656-19-6*0 Hypobromite [BrO]- (g) -96.13 -103.61 ± 0.53 kJ/mol 95.9039 ± 14380-62-2*0 Ammonium [NH4]+ (g) 643.02 631.71 ± 0.21 kJ/mol 18.03795 ± 14798-03-9*0 (E)-Diazene HNNH (g, trans) 207.12 199.98 ± 0.41 kJ/mol 30.02936 ± 15626-43-4*0 Peroxynitrous acid HOONO (g, cis, cis) -5.80 -14.24 ± 0.36 kJ/mol 63.01288 ± 14691-52-2*3 Ethylidene CH3CH (g, singlet) 373.48 366.25 ± 0.65 kJ/mol 28.0532 ± 4218-50-2*2 Cyanogen NCCN (g) 308.17 310.12 ± 0.41 kJ/mol 52.0349 ± 460-19-5*0 1-Butene CH2CHCH2CH3 (g) 21.04 0.09 ± 0.36 kJ/mol 56.1063 ± 106-98-9*0 n-Pentane CH3CH2CH2CH2CH3 (g) -114.37 -146.25 ± 0.31 kJ/mol 72.1488 ± 109-66-0*0 n-Hexane CH3CH2CH2CH2CH2CH3 (g) -130.02 -166.89 ± 0.34 kJ/mol 86.1754 ± 110-54-3*0 Trimethylamine (CH3)3N (g) 2.11 -26.74 ± 0.76 kJ/mol 59.1103 ± 75-50-3*0 Dichloromethylene CCl2 (g, singlet) 229.51 230.69 ± 0.64 kJ/mol 82.9161 ± 1605-72-7*2 Trifluoromethylium [CF3]+ (g) 409.53 406.32 ± 0.46 kJ/mol 69.00536 ± 18851-76-8*0 Hydrogen fluoride HF (aq) -334.50 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*800 Ethyl nitrite CH3CH2ONO (g, trans-cis) -81.38 -100.12 ± 0.88 kJ/mol 75.0666 ± 109-95-5*1 Water H2O (l) -285.826 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*590 Azido radical NNN (g) 452.28 449.70 ± 0.60 kJ/mol 42.02022 ± 12596-60-0*0 Nitrous oxide NNO (g) 86.000 82.571 ± 0.097 kJ/mol 44.01288 ± 10024-97-2*0 Hydroxyoxomethylium [HOCO]+ (g) 600.66 597.57 ± 0.44 kJ/mol 45.0169 ± 638-71-1*0 Hydroxyamidogen HNOH (g, trans) 101.37 94.53 ± 0.81 kJ/mol 32.02202 ± 13940-32-4*1 Hydrazoic acid HNNN (g) 297.93 291.63 ± 0.49 kJ/mol 43.02816 ± 7782-79-8*0 Ethylidene CH3CH (g, triplet) 361.20 354.20 ± 0.51 kJ/mol 28.0532 ± 4218-50-2*1 Vinylidene CCH2 (g) 411.19 412.10 ± 0.30 kJ/mol 26.0373 ± 2143-69-3*0 Acetylium [CH3CO]+ (g) 666.59 659.31 ± 0.44 kJ/mol 43.0441 ± 15762-07-9*0 Hydroxymethylium [CH2OH]+ (g) 717.68 709.74 ± 0.18 kJ/mol 31.03337 ± 18682-95-6*0 Fluoroacetylene HCCF (g) 104.96 105.67 ± 0.40 kJ/mol 44.0277 ± 2713-09-9*0 Peroxyformyl HC(O)OO (g, syn) -100.37 -105.67 ± 0.92 kJ/mol 61.0168 ± 56240-83-6*1 trans-2-Butene CH3CHCHCH3 (g, trans) 9.42 -11.14 ± 0.38 kJ/mol 56.1063 ± 624-64-6*0 1,2-Butadiene CH3CHCCH2 (g) 175.91 162.41 ± 0.46 kJ/mol 54.0904 ± 590-19-2*0 Trioxidane HOOOH (g, trans) -82.01 -90.85 ± 0.63 kJ/mol 50.01408 ± 14699-99-1*1 Ketenyl HCCO (g) 177.06 178.16 ± 0.55 kJ/mol 41.0287 ± 51095-15-9*0 Formic acid HC(O)OH (g) -371.09 -378.38 ± 0.22 kJ/mol 46.0254 ± 64-18-6*0 Dioxidanide [HO2]- (g) -88.87 -91.53 ± 0.35 kJ/mol 33.00729 ± 14691-59-9*0 Acetic acid CH3C(O)OH (g, anti) -397.81 -411.59 ± 0.53 kJ/mol 60.0520 ± 64-19-7*2 Methyl formate HC(O)OCH3 (g, anti) -326.7 -339.1 ± 1.1 kJ/mol 60.0520 ± 107-31-3*2 Trichloromethyl CCl3 (g) 72.01 71.54 ± 0.84 kJ/mol 118.3688 ± 3170-80-7*0 1,1-Dichloroethane CH3CHCl2 (g) -120.29 -133.07 ± 0.44 kJ/mol 98.9586 ± 75-34-3*0 Difluorodioxidane FOOF (g) 35.31 31.58 ± 0.41 kJ/mol 69.99561 ± 7783-44-0*0 Bromoform CHBr3 (g) 75.7 49.6 ± 1.3 kJ/mol 252.7306 ± 75-25-2*0 Bromobenzene C6H5Br (g) 126.7 104.6 ± 1.3 kJ/mol 157.0079 ± 108-86-1*0 Benzaldehyde C6H5C(O)H (g) -19.28 -37.07 ± 0.91 kJ/mol 106.1219 ± 100-52-7*0 Propionaldehyde CH3CH2CHO (g) -171.36 -186.77 ± 0.25 kJ/mol 58.0791 ± 123-38-6*0 Nitrosomethane CH3NO (g) 80.78 70.87 ± 0.91 kJ/mol 45.04066 ± 865-40-7*0 Isocyanoacetylene HCCNC (g) 484.7 486.9 ± 1.0 kJ/mol 51.0468 ± 66723-45-3*0 Silanone SiH2O (g) -93.6 -98.9 ± 1.1 kJ/mol 46.10078 ± 22755-01-7*0 Silyloxy SiH3O (g) 8.5 0.5 ± 1.5 kJ/mol 47.10872 ± 81429-20-1*0 Chlorous acid HOClO (g) 25.79 20.74 ± 0.63 kJ/mol 68.4594 ± 13898-47-0*0 Iodooxidanyl IO (g) 126.99 125.06 ± 0.82 kJ/mol 142.90387 ± 14696-98-1*0 Carbon monoxide cation [CO]+ (g) 1238.306 1241.585 ± 0.026 kJ/mol 28.00955 ± 12144-04-6*0 Azanide [NH2]- (g) 114.82 111.94 ± 0.29 kJ/mol 16.02317 ± 17655-31-1*0 Hydrazine NH2NH2 (g) 111.72 97.57 ± 0.42 kJ/mol 32.04524 ± 302-01-2*0 Aminooxy NH2O (g) 70.93 64.32 ± 0.71 kJ/mol 32.02202 ± 13408-29-2*0 Nitrate [ON(O)O]- (g) -300.33 -306.88 ± 0.60 kJ/mol 62.00549 ± 14797-55-8*0 Fluorodioxidanyl FOO (g) 26.88 25.17 ± 0.26 kJ/mol 50.99720 ± 15499-23-7*0 Cyanogen iodide ICN (g) 222.6 222.0 ± 1.6 kJ/mol 152.92191 ± 506-78-5*0 Formyl anion [HCO]- (g) 11.60 11.98 ± 0.44 kJ/mol 29.01859 ± 57340-31-5*0 1-Methylethenyl CH3CCH2 (g) 263.07 252.68 ± 0.67 kJ/mol 41.0718 ± 15552-77-9*0 1,2-Propadienyl anion [CH2CCH]- (g) 265.76 262.70 ± 0.67 kJ/mol 39.0565 ± 64066-06-4*0 2-Propynylidyne CCCH (g) 718.01 722.36 ± 0.80 kJ/mol 37.0400 ± 53590-28-6*0 neo-Pentane C(CH3)4 (g) -134.52 -167.39 ± 0.39 kJ/mol 72.1488 ± 463-82-1*0 Hypofluorite [FO]- (g) -108.84 -108.67 ± 0.48 kJ/mol 34.99835 ± 12763-66-5*0 Ethoxy CH3CH2O (g, A 2A') 5.42 -9.02 ± 0.45 kJ/mol 45.0605 ± 2154-50-9*52 Dicarbon monoxide CCO (g, triplet) 377.12 381.16 ± 0.83 kJ/mol 40.0208 ± 12071-23-7*1 1-Propanol CH3CH2CH2OH (g) -231.40 -255.24 ± 0.25 kJ/mol 60.0950 ± 71-23-8*0 Chloromethylene CHCl (g, singlet) 320.27 320.55 ± 0.89 kJ/mol 48.4713 ± 2108-20-5*2 Dibromomethane CH2Br2 (g) 26.0 4.6 ± 1.1 kJ/mol 173.8346 ± 74-95-3*0 1,1-Difluoroethene CH2CF2 (g) -343.58 -350.64 ± 0.77 kJ/mol 64.0341 ± 75-38-7*0 3-Buten-2-one CH3C(O)CHCH2 (g, trans) -95.49 -111.73 ± 0.87 kJ/mol 70.0898 ± 78-94-4*1 Urea NH2C(O)NH2 (g) -218.22 -234.62 ± 0.42 kJ/mol 60.05534 ± 57-13-6*0 Oxomethaniminium [NH2CO]+ (g) 695.30 688.74 ± 0.90 kJ/mol 44.03217 ± 59348-22-0*0 Acetamide CH3C(O)NH2 (g) -219.62 -237.13 ± 0.57 kJ/mol 59.0672 ± 60-35-5*0 Cyclobutadiene CH(CHCHCH) (g) 436.50 428.05 ± 0.79 kJ/mol 52.0746 ± 1120-53-2*0 1,3-Hexadiyne HCCCCCH2CH3 (g) 405.04 393.34 ± 0.98 kJ/mol 78.1118 ± 4447-21-6*0 Iodine monochloride ICl (g) 19.024 17.391 ± 0.013 kJ/mol 162.35717 ± 7790-99-0*0 Iodotrifluoromethane CF3I (g) -583.45 -589.33 ± 0.49 kJ/mol 195.91038 ± 2314-97-8*0 Trinitrogen cation [NNN]+ (g) 1519.44 1516.81 ± 0.89 kJ/mol 42.01967 ± 12185-03-4*0 Oxonitrate anion [NO]- (g) 88.11 88.14 ± 0.25 kJ/mol 30.00669 ± 14967-78-3*0 Nitrosyl ion [NO]+ (g) 984.499 984.494 ± 0.066 kJ/mol 30.00559 ± 14452-93-8*0 Nitrogen sesquioxide ONN(O)O (g) 90.75 86.18 ± 0.15 kJ/mol 76.01168 ± 10544-73-7*0 Methylene anion [CH2]- (g) 328.17 328.60 ± 0.18 kJ/mol 14.02713 ± 50928-07-9*0 Methylidyne anion [CH]- (g) 475.74 479.07 ± 0.22 kJ/mol 13.01919 ± 28604-54-8*0 Ethanium [C2H7]+ (g, bridged C2) 874.32 858.05 ± 0.62 kJ/mol 31.0764 ± 24669-33-8*1 Hydrogen isocyanide HNC (g) 192.05 192.43 ± 0.33 kJ/mol 27.02538 ± 6914-07-4*0 Hydroxymethylene HCOH (g, trans) 112.68 108.91 ± 0.27 kJ/mol 30.02598 ± 19710-56-6*1 Carbonic acid C(O)(OH)2 (g, cis-cis) -602.73 -612.90 ± 0.75 kJ/mol 62.0248 ± 463-79-6*1 Peroxyformate [HC(O)OO]- (g, syn) -340.91 -346.36 ± 0.95 kJ/mol 61.0174 ± 104960-05-6*1 Isoprene CH2C(CH3)CHCH2 (g) 96.12 75.60 ± 0.67 kJ/mol 68.1170 ± 78-79-5*0 1-Hydroxyethylium [CH3CHOH]+ (g, anti) 608.75 594.32 ± 0.39 kJ/mol 45.0600 ± 18682-96-7*1 Chlorine trifluoride FCl(F)F (g) -158.6 -162.7 ± 1.2 kJ/mol 92.44791 ± 7790-91-2*0 Ethynol HCCOH (g, singlet) 95.31 92.82 ± 0.80 kJ/mol 42.0367 ± 32038-79-2*2 Formyloxidanyl HC(O)O (g, 2A1) -125.90 -127.56 ± 0.56 kJ/mol 45.0174 ± 16499-21-1*1 Chloronium [HClH]+ (g) 881.3 878.2 ± 1.1 kJ/mol 37.46803 ± 36658-55-6*0 Glyoxal OCHCHO (g, trans) -207.71 -213.35 ± 0.61 kJ/mol 58.0361 ± 107-22-2*1 1,1,1-Trichloroethane CH3CCl3 (g) -134.33 -144.90 ± 0.48 kJ/mol 133.4033 ± 71-55-6*0 Tetrachloroethene CCl2CCl2 (g) -22.3 -23.2 ± 1.1 kJ/mol 165.8322 ± 127-18-4*0 Chlorodifluoromethane CHClF2 (g) -476.01 -482.21 ± 0.98 kJ/mol 86.4681 ± 75-45-6*0 Fluoromethylene CHF (g, singlet) 148.39 148.67 ± 0.43 kJ/mol 32.01704 ± 13453-52-6*2 Fluoroformyl FCO (g) -176.61 -176.03 ± 0.37 kJ/mol 47.00850 ± 1871-24-5*0 2-Bromoethanol BrCH2CH2OH (g) -196.15 -220.21 ± 0.53 kJ/mol 124.9645 ± 540-51-2*0 Carbon suboxide OCCCO (g) -94.9 -92.8 ± 1.2 kJ/mol 68.0309 ± 504-64-3*0 Benzyl C6H5CH2 (g) 230.05 211.24 ± 0.56 kJ/mol 91.1305 ± 2154-56-5*0 Phenoxy C6H5O (g) 70.87 55.58 ± 0.84 kJ/mol 93.1033 ± 2122-46-5*0 1-Cyanovinyl CH2CCN (g) 419.0 418.9 ± 1.1 kJ/mol 52.0547 ± 15091-87-9*0 Cyanomethylene HCCN (g, triplet) 479.9 483.3 ± 1.1 kJ/mol 39.0361 ± 2612-62-6*1 3-Methylenecyclopropene CH2C(CHCH) (g) 395.82 387.88 ± 0.85 kJ/mol 52.0746 ± 4095-06-1*0 Phenylperoxy C6H5OO (g) 153.1 136.1 ± 1.4 kJ/mol 109.1027 ± 91422-02-5*0 Trimethylsilane SiH(CH3)3 (g) -124.6 -150.7 ± 1.6 kJ/mol 74.1970 ± 993-07-7*0 Hydrogen bromide HBr (aq) -120.59 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*800 Hydrogen peroxide cation [H2O2]+ (g, trans) 897.23 890.25 ± 0.51 kJ/mol 34.01413 ± 12325-10-9*1 Bromonium [HBrH]+ (g) 925.3 914.6 ± 1.5 kJ/mol 81.9193 ± 36658-54-5*0 Methanide [CH3]- (g) 141.10 137.66 ± 0.24 kJ/mol 15.03507 ± 15194-58-8*0 Methyliumyl [CH2]+ (g) 1393.19 1394.05 ± 0.11 kJ/mol 14.02603 ± 15091-72-2*0 Ethylidyne CH3C (g, quartet) 640.7 636.1 ± 1.1 kJ/mol 27.0452 ± 67624-57-1*2 Aminomethylium [CH2NH2]+ (g) 763.98 752.01 ± 0.64 kJ/mol 30.04865 ± 488821-23-4*0 Isocyanomethanide [CH2NC]- (g) 254.04 251.00 ± 0.96 kJ/mol 40.0446 ± 81704-80-5*0 1-Methylethylidene CH3CCH3 (g, singlet) 315.91 302.01 ± 0.99 kJ/mol 42.0797 ± 40852-89-9*2 1-Butyne CH3CH2CCH (g) 180.26 166.68 ± 0.67 kJ/mol 54.0904 ± 107-00-6*0 Trioxidanide [HOOO]- (g, gauche) -120.0 -123.1 ± 1.6 kJ/mol 49.00669 ± 89965-38-8*2 iso-Pentane CH3CH2CH(CH3)2 (g) -119.12 -153.17 ± 0.43 kJ/mol 72.1488 ± 78-78-4*0 Cyclohexane CH2(CH2CH2CH2CH2CH2) (g) -83.37 -122.94 ± 0.30 kJ/mol 84.1595 ± 110-82-7*0 1-Hydroxyethyl CH3CHOH (g, gauche-syn) -41.35 -54.82 ± 0.79 kJ/mol 45.0605 ± 2348-46-1*2 Protonated oxirane [(CH2CH2)OH]+ (g) 725.57 709.40 ± 0.62 kJ/mol 45.0600 ± *38607-31-7*0 Methanediol CH2(OH)2 (g, conrot gauche) -379.36 -393.56 ± 0.92 kJ/mol 48.0413 ± 463-57-0*1 Methoxyethane CH3CH2OCH3 (g) -194.43 -217.32 ± 0.58 kJ/mol 60.0950 ± 540-67-0*0 Cyanic acid HOCN (g) -12.34 -15.06 ± 0.43 kJ/mol 43.02478 ± 420-05-3*0 Fulminic acid HCNO (g) 170.70 169.25 ± 0.44 kJ/mol 43.02478 ± 51060-05-0*0 Isofulminic acid HONC (g) 235.22 233.52 ± 0.43 kJ/mol 43.02478 ± 506-85-4*0 Nitrosomethylidyne CNO (g) 389.3 390.7 ± 1.1 kJ/mol 42.01684 ± 82267-15-0*0 Acetic acid CH3C(O)OH (g) -418.61 -432.74 ± 0.38 kJ/mol 60.0520 ± 64-19-7*0 Hydrogen chloride HCl (aq) -166.992 ± 0.023 kJ/mol 36.46064 ± 7647-01-0*800 Acetyl chloride CH3C(O)Cl (g) -232.58 -241.57 ± 0.33 kJ/mol 78.4973 ± 75-36-5*0 Chlorohydroxymethyl CH(Cl)OH (g, syn-gauche) -54.7 -60.8 ± 1.1 kJ/mol 65.4787 ± 147139-06-8*1 1,1-Dichloroethene CH2CCl2 (g) 8.94 2.95 ± 0.49 kJ/mol 96.9427 ± 75-35-4*0 Dichlorodifluoromethane CF2Cl2 (g) -489.47 -493.65 ± 0.67 kJ/mol 120.9129 ± 75-71-8*0 Fluorodichloromethane CHFCl2 (g) -278.14 -283.59 ± 0.91 kJ/mol 102.9224 ± 75-43-4*0 Difluoromethylene CF2 (g, singlet) -193.89 -193.42 ± 0.36 kJ/mol 50.00751 ± 2154-59-8*2 Fluoromethylene CHF (g, triplet) 210.36 210.70 ± 0.66 kJ/mol 32.01704 ± 13453-52-6*1 Formyl fluoride CHFO (g) -378.63 -382.22 ± 0.31 kJ/mol 48.01644 ± 1493-02-3*0 Propyl nitrite CH3CH2CH2ONO (g, gauche-trans-cis) -96.1 -121.1 ± 1.8 kJ/mol 89.0932 ± 543-67-9*1 2-Cyanovinyl CHCHCN (g, syn) 448.0 445.5 ± 1.2 kJ/mol 52.0547 ± 189141-20-6*2 1,2,3-Butatrien-1-ylium [CH2CCCH]+ (g, singlet) 1271.7 1269.9 ± 1.1 kJ/mol 51.0661 ± 81932-79-8*2 1-Buten-3-yn-1-yl HCCCHCH (g, trans) 547.17 544.51 ± 0.88 kJ/mol 51.0666 ± 2810-61-9*1 Silyl SiH3 (g) 204.6 199.2 ± 1.1 kJ/mol 31.10932 ± 13765-44-1*0 Hydroxysilyl SiH2OH (g) -104.2 -112.1 ± 1.6 kJ/mol 47.10872 ± 113648-09-2*0 Trimethylsilanol (CH3)3SiOH (g) -464.5 -490.8 ± 1.9 kJ/mol 90.1964 ± 1066-40-6*0 2-Propanamine CH3CH(CH3)NH2 (g) -56.78 -83.57 ± 0.53 kJ/mol 59.1103 ± 75-31-0*0 Hypoiodite [IO]- (g) -102.46 -104.27 ± 0.88 kJ/mol 142.90442 ± 15065-65-3*0 Dinitrogen cation [N2]+ (g) 1503.311 1503.312 ± 0.000 kJ/mol 28.01293 ± 13966-04-6*0 Carbon dioxide cation [CO2]+ (g) 936.091 936.926 ± 0.017 kJ/mol 44.00895 ± 12181-61-2*0 Ammonium [NH4]+ (aq) -133.075 ± 0.056 kJ/mol 18.03795 ± 14798-03-9*800 Hydrazino NH2NH (g) 234.77 224.25 ± 0.69 kJ/mol 31.03730 ± 13598-46-4*0 Methylamidogen CH3NH (g) 187.58 176.93 ± 0.46 kJ/mol 30.04920 ± 15622-51-2*0 Methyleneamidogen CH2N (g) 242.00 238.35 ± 0.58 kJ/mol 28.03332 ± 15845-29-1*0 (E)-Iminomethyl HCNH (g, trans) 275.79 272.18 ± 0.61 kJ/mol 28.03332 ± 54980-10-8*0 Hydrogen isocyanide cation [HNC]+ (g) 1351.51 1351.47 ± 0.84 kJ/mol 27.02483 ± 74158-11-5*0 Chloroacetylene HCCCl (g) 228.13 228.99 ± 0.70 kJ/mol 60.4820 ± 593-63-5*0 Dichloride [Cl2]- (g) -229.8 -228.7 ± 1.4 kJ/mol 70.9059 ± 12595-89-0*0 iso-Propylium [CH3CHCH3]+ (g) 822.88 805.80 ± 0.24 kJ/mol 43.0871 ± 19252-53-0*0 Chlorine atom cation Cl+ (g) 1370.806 1372.602 ± 0.0011 kJ/mol 35.45215 ± 24203-47-2*0 n-Propylide [CH3CH2CH2]- (g) 122.9 105.3 ± 1.2 kJ/mol 43.0882 ± 59513-13-2*0 Hydroperoxonium [H2OOH]+ (g, trans) 738.1 727.8 ± 1.2 kJ/mol 35.02207 ± 70850-26-9*1 1-Propenyl CH3CHCH (g, cis) 278.31 267.47 ± 0.75 kJ/mol 41.0718 ± 6067-68-1*2 Propargylenide [HCCCH]- (g, transoid 2B) 434.4 436.9 ± 1.1 kJ/mol 38.0485 ± 82906-05-6*1 t-Butyl (CH3)3C (g) 75.08 49.78 ± 0.77 kJ/mol 57.1143 ± 1605-73-8*0 Cyclobutane CH2(CH2CH2CH2) (g) 52.35 27.81 ± 0.39 kJ/mol 56.1063 ± 287-23-0*0 Cyclobutene CH2(CH2CHCH) (g) 177.65 160.60 ± 0.71 kJ/mol 54.0904 ± 822-35-5*0 2-Butynyl CH3CCCH2 (g) 318.07 310.70 ± 0.79 kJ/mol 53.0825 ± 82252-88-8*0 2-Hydroxyethyl CH2CH2OH (g, gauche-anti) -10.84 -23.47 ± 0.82 kJ/mol 45.0605 ± 4422-54-2*2 Ketene CH2CO (g, triplet) 182.0 177.0 ± 1.1 kJ/mol 42.0367 ± 463-51-4*1 Chloro hypochlorite ClOCl (g) 79.78 77.97 ± 0.36 kJ/mol 86.9048 ± 7791-21-1*0 Oxoethylidene HCC(O)H (g, syn-triplet) 261.1 257.6 ± 1.1 kJ/mol 42.0367 ± 39920-84-8*12 Methanediol CH2(OH)2 (g, disrot gauche) -369.05 -382.21 ± 0.95 kJ/mol 48.0413 ± 463-57-0*2 Methoxyethene CH2CHOCH3 (g, cis) -89.3 -107.1 ± 1.1 kJ/mol 58.0791 ± 107-25-5*2 Methyl acetate CH3C(O)OCH3 (g, anti) -363.4 -382.1 ± 1.2 kJ/mol 74.0785 ± 79-20-9*2 Carboxymethyl CH2C(O)OH (g, syn) -227.2 -237.0 ± 1.2 kJ/mol 59.0440 ± 2887-46-9*1 (Methylamino)methyl CH3NHCH2 (g) 167.93 151.48 ± 0.95 kJ/mol 44.0758 ± 31277-24-4*0 Dimethylamidogen (CH3)2N (g) 173.9 156.6 ± 1.5 kJ/mol 44.0758 ± 15337-44-7*0 Cyanoamidogen HNCN (g) 322.94 320.45 ± 0.84 kJ/mol 41.03212 ± 12347-01-2*0 Bromotrichloromethane CCl3Br (g) -32.75 -41.89 ± 0.59 kJ/mol 198.2728 ± 75-62-7*0 Chloromethylidyne CCl (g) 430.43 434.18 ± 0.67 kJ/mol 47.4634 ± 3889-76-7*0 Chlorine pentafluoride FCl(F)(F)(F)F (g) -226.1 -234.2 ± 1.7 kJ/mol 130.44472 ± 13637-63-3*0 Chloromethanol CH2(Cl)OH (g) -232.2 -242.5 ± 1.2 kJ/mol 66.4866 ± 15454-33-8*0 Chloromethoxy CH2(Cl)O (g) -11.9 -18.7 ± 1.1 kJ/mol 65.4787 ± 114282-89-2*0 Tetraiodomethane CI4 (g) 332.2 327.1 ± 4.1 kJ/mol 519.62858 ± 507-25-5*0 Fluorotrichloromethane CCl3F (g) -285.28 -288.43 ± 0.78 kJ/mol 137.3672 ± 75-69-4*0 Chlorooxy hypochlorite ClOOCl (g) 134.56 131.33 ± 0.56 kJ/mol 102.9042 ± 12292-23-8*0 2-Chloroethanol ClCH2CH2OH (g) -249.80 -266.52 ± 0.59 kJ/mol 80.5132 ± 107-07-3*0 o-Benzyne C6H4 (g, singlet) 468.12 459.20 ± 0.87 kJ/mol 76.0960 ± 462-80-6*2 Tropyl CH(CHCHCHCHCHCH) (g) 295.8 278.4 ± 1.1 kJ/mol 91.1305 ± 3551-27-7*0 Iodobenzene C6H5I (g) 177.82 161.83 ± 0.99 kJ/mol 204.0084 ± 591-50-4*0 Acetonyl CH3C(O)CH2 (g) -19.01 -31.72 ± 0.98 kJ/mol 57.0712 ± 3122-07-4*0 Trifluoroethene CF2CHF (g) -493.1 -498.1 ± 1.6 kJ/mol 82.0245 ± 359-11-5*0 Isocyanoethene CH2CHNC (g) 285.14 278.64 ± 0.94 kJ/mol 53.0627 ± 14668-82-7*0 1-Isocyanoethylidene CH3CNC (g, singlet) 527.8 523.1 ± 1.1 kJ/mol 53.0627 ± *498544-34-6*2 3-Methylene-1-cyclopropen-1-yl CH2C(CHC) (g) 628.1 625.5 ± 1.1 kJ/mol 51.0666 ± 127611-24-9*0 Peroxyhypochlorous acid HOOCl (g) 3.95 -1.32 ± 0.95 kJ/mol 68.4594 ± 67952-07-2*0 Fulvene CH2C(CHCHCHCH) (g) 231.9 215.8 ± 1.1 kJ/mol 78.1118 ± 497-20-1*0 Oxosilylene SiO (g) -98.11 -96.95 ± 0.65 kJ/mol 44.08490 ± 10097-28-6*0 Silyloxy anion [SiH3O]- (g) -311.7 -321.1 ± 1.6 kJ/mol 47.10927 ± 43336-62-5*0 1-Propanamine CH3CH2CH2NH2 (g) -42.36 -70.10 ± 0.42 kJ/mol 59.1103 ± 107-10-8*0 Perchloryl OCl(O)O (g) 191.82 186.15 ± 0.62 kJ/mol 83.4509 ± 13932-10-0*0 Dioxodiazoxane ONONO (g, t, t) 93.0 87.9 ± 1.3 kJ/mol 76.01168 ± 122413-35-8*1 Nitric acid HON(O)O (aq) -206.64 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*800 Isodiazene NNH2 (g) 307.54 300.46 ± 0.63 kJ/mol 30.02936 ± 28647-38-3*0 Oxoammoniumyl [HNO]+ (g) 1092.47 1089.54 ± 0.67 kJ/mol 31.01353 ± 63559-87-5*0 Nitryl hydride HN(O)O (g) -36.6 -43.6 ± 1.1 kJ/mol 47.01348 ± 90266-90-3*0 Peroxynitrous acid HOONO (g, trans, perp) 7.78 0.70 ± 0.41 kJ/mol 63.01288 ± 14691-52-2*1 Carbon anion C- (g) 589.621 594.768 ± 0.046 kJ/mol 12.01125 ± 14337-00-9*0 Chlorate [OCl(O)O]- (g) -207.2 -212.7 ± 1.6 kJ/mol 83.4514 ± 14866-68-3*0 Ethylidyne CH3C (g) 508.53 504.83 ± 0.80 kJ/mol 27.0452 ± 67624-57-1*0 Ethynylium [CCH]+ (g) 1687.59 1690.93 ± 0.16 kJ/mol 25.0288 ± 16456-59-0*0 Methylamidogen anion [CH3NH]- (g) 145.35 134.52 ± 0.65 kJ/mol 30.04975 ± 54448-39-4*0 Iminomethylium [HCNH]+ (g) 953.3 949.3 ± 1.4 kJ/mol 28.03277 ± 196216-36-1*0 Hydrogen fluoride HF (aq, 20 H2O) -321.05 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*818 Methylperoxy anion [CH3OO]- (g) -89.81 -99.96 ± 0.58 kJ/mol 47.0339 ± 35683-44-4*0 Propylidyne CH3CH2C (g, doublet) 492.5 482.8 ± 1.2 kJ/mol 41.0718 ± 85056-54-8*1 Trioxidane HOOOH (g, cis) -72.74 -81.23 ± 0.82 kJ/mol 50.01408 ± 14699-99-1*2 Cyclopropylidene C(CH2CH2) (g, singlet) 474.87 466.84 ± 0.85 kJ/mol 40.0639 ± 2143-70-6*1 Propargylium [CH2CCH]+ (g) 1193.42 1190.60 ± 0.33 kJ/mol 39.0554 ± 21540-27-2*0 Cycloprop-1-enyl CH2(CHC) (g) 527.35 522.96 ± 0.59 kJ/mol 39.0559 ± 19528-44-0*0 Propargylenide [HCCCH]- (g, cisoid 2A) 436.9 439.4 ± 1.1 kJ/mol 38.0485 ± 82906-05-6*2 2-Propyn-1-ylium-1-ylidene [CCCH]+ (g) 1598.1 1602.5 ± 1.3 kJ/mol 37.0395 ± 75104-46-0*0 Chloric acid HOCl(O)O (g) 2.2 -4.9 ± 1.6 kJ/mol 84.4588 ± 7790-93-4*0 Cyclopentene CH2(CH2CHCHCH2) (g) 59.43 35.17 ± 0.47 kJ/mol 68.1170 ± 142-29-0*0 n-Heptane CH3(CH2)5CH3 (g) -145.58 -187.46 ± 0.46 kJ/mol 100.2019 ± 142-82-5*0 n-Octane CH3(CH2)6CH3 (g) -161.25 -208.08 ± 0.54 kJ/mol 114.2285 ± 111-65-9*0 Difluoride [F2]- (g) -291.0 -290.2 ± 1.5 kJ/mol 37.9973550 ± 12184-85-9*0 2-Hydrotrioxy OO(H)O (g) 299.6 293.9 ± 1.2 kJ/mol 49.00614 ± *12500-78-6*0 Ethenol CH2CHOH (g, anti) -108.73 -119.44 ± 0.60 kJ/mol 44.0526 ± 557-75-5*2 Vinoxy CH2CHO (g) 22.97 16.13 ± 0.68 kJ/mol 43.0446 ± 6912-06-7*0 Oxoethanide [CH3CO]- (g) -44.0 -49.5 ± 1.3 kJ/mol 43.0452 ± 78944-68-0*0 Oxirene O(CHCH) (g, singlet) 273.18 271.02 ± 0.92 kJ/mol 42.0367 ± 157-18-6*2 Hydroxyformyl HOCO (g, cis) -174.31 -177.34 ± 0.50 kJ/mol 45.0174 ± 2564-86-5*2 Hydroxyformyl anion [HOCO]- (g, cis) -319.53 -322.11 ± 0.57 kJ/mol 45.0180 ± 78944-70-4*2 Ethoxyethane CH3CH2OCH2CH3 (g) -222.99 -250.41 ± 0.82 kJ/mol 74.1216 ± 60-29-7*0 Acetone enol CH3C(OH)CH2 (g, syn) -152.0 -169.4 ± 1.1 kJ/mol 58.0791 ± 29456-04-0*1 Acetate [CH3C(O)O]- (g) -496.4 -505.5 ± 1.2 kJ/mol 59.0446 ± 71-50-1*0 (Dimethylamino)methyl (CH3)2NCH2 (g) 165.8 142.8 ± 1.3 kJ/mol 58.1024 ± 30208-47-0*0 Chloromethide [CH2Cl]- (g) 56.4 53.2 ± 2.0 kJ/mol 49.4798 ± 60291-29-4*0 Hexachloroethane CCl3CCl3 (g) -147.0 -149.4 ± 1.3 kJ/mol 236.7376 ± 67-72-1*0 cis-1,2-Dichloroethene CHClCHCl (g, cis) 3.47 -2.53 ± 0.54 kJ/mol 96.9427 ± 156-59-2*0 Bromanylium Br+ (g) 1257.778 1251.715 ± 0.057 kJ/mol 79.90345 ± 22541-56-6*0 Dichloroacetylene ClCCCl (g) 230.13 233.44 ± 0.94 kJ/mol 94.9268 ± 7572-29-4*0 Difluoromethylene CF2 (g) -193.89 -193.42 ± 0.36 kJ/mol 50.00751 ± 2154-59-8*0 Hexafluoroethane CF3CF3 (g) -1334.14 -1342.44 ± 0.97 kJ/mol 138.0118 ± 76-16-4*0 1,1-Difluoroethane CH3CHF2 (g) -489.02 -502.74 ± 0.55 kJ/mol 66.0500 ± 75-37-6*0 2-Fluoroethanol FCH2CH2OH (g) -405.86 -423.10 ± 0.99 kJ/mol 64.0589 ± 371-62-0*0 Fluoronium [HFH]+ (g, singlet) 774.10 771.16 ± 0.88 kJ/mol 21.01373 ± 12206-67-6*2 Benzylide [C6H5CH2]- (g) 141.92 123.01 ± 0.42 kJ/mol 91.1310 ± 18860-15-6*0 Ethylbenzene C6H5CH2CH3 (g) 58.20 29.73 ± 0.55 kJ/mol 106.1650 ± 100-41-4*0 Phenylethene C6H5CHCH2 (g) 169.68 148.34 ± 0.55 kJ/mol 104.1491 ± 100-42-5*0 Phenylacetylene C6H5CCH (g) 332.4 317.2 ± 1.1 kJ/mol 102.1332 ± 536-74-3*0 Nitrogen atom cation N+ (g) 1872.907 1875.689 ± 0.024 kJ/mol 14.006191 ± 14158-23-7*0 Acetonate [CH3C(O)CH2]- (g) -187.81 -201.36 ± 0.98 kJ/mol 57.0717 ± 24262-31-5*0 Methyl nitrite CH3ONO (g) -55.52 -66.19 ± 0.45 kJ/mol 61.0401 ± 624-91-9*0 Propadienone CH2CCO (g) 131.92 130.29 ± 0.79 kJ/mol 54.0474 ± 61244-93-7*0 1-Cyanoethylidene CH3CCN (g, singlet) 475.6 470.5 ± 1.1 kJ/mol 53.0627 ± 498544-34-6*2 3-Imino-2-propen-1-ylidene CHCHCNH (g, syn-singlet) 530.6 525.8 ± 1.2 kJ/mol 53.0627 ± 916765-05-4*11 2-(Methylidyneamino)ethenyl CHCHNCH (g, anti-singlet) 600.6 595.6 ± 1.2 kJ/mol 53.0627 ± 123416-74-0*11 1-Cyanovinyl anion [CH2CCN]- (g) 224.3 221.5 ± 1.3 kJ/mol 52.0553 ± 36259-97-9*0 2-Cyanovinyl CHCHCN (g, anti) 448.0 445.5 ± 1.1 kJ/mol 52.0547 ± 189141-20-6*1 3-Buten-1-ynyl CH2CHCC (g) 628.4 626.2 ± 1.1 kJ/mol 51.0666 ± 108179-98-2*0 Water dimer (H2O)2 (g) -491.077 -499.117 ± 0.051 kJ/mol 36.03056 ± 25655-83-8*0 Methylsilane CH3SiH3 (g) -9.46 -25.63 ± 0.96 kJ/mol 46.14384 ± 992-94-9*0 Trimethylsiloxy (CH3)3SiO (g) -187.4 -210.4 ± 2.0 kJ/mol 89.1885 ± 54572-10-0*0 1,5-Hexadiyne HCCCH2CH2CCH (g) 431.5 421.0 ± 1.2 kJ/mol 78.1118 ± 628-16-0*0 N-Methylethanamine CH3CH2NHCH3 (g) -17.8 -45.9 ± 1.1 kJ/mol 59.1103 ± 624-78-2*0 Naphthalene C6H4(C4H4) (g) 170.97 147.49 ± 0.59 kJ/mol 128.1705 ± 91-20-3*0 Trioxygen difluoride FOOOF (g, trans) 112.3 106.5 ± 1.1 kJ/mol 85.99501 ± 16829-28-0*1 Carbon cation C+ (g) 1797.851 1803.449 ± 0.045 kJ/mol 12.01015 ± 14067-05-1*0 Methyliumylidene [CH]+ (g) 1619.755 1623.099 ± 0.052 kJ/mol 13.01809 ± 24361-82-8*0 Ethylidyne CH3C (g, doublet) 508.53 504.83 ± 0.80 kJ/mol 27.0452 ± 67624-57-1*1 Vinylidene anion [CCH2]- (g) 363.99 363.80 ± 0.50 kJ/mol 26.0378 ± 66295-36-1*0 Ozonide [OOO]- (g) -58.48 -60.84 ± 0.21 kJ/mol 47.99875 ± 12596-80-4*0 Bromochlorane BrCl (g) 21.882 14.439 ± 0.060 kJ/mol 115.3567 ± 13863-41-7*0 Oxidanylium [OH]+ (g) 1293.192 1293.221 ± 0.035 kJ/mol 17.00679 ± 12259-29-9*0 Cyanomethanide [CH2CN]- (g) 114.24 111.41 ± 0.68 kJ/mol 40.0446 ± 21438-99-3*0 Dioxaziridinyl N(OO) (g) 355.1 352.6 ± 1.4 kJ/mol 46.00554 ± 66252-29-7*0 Methanol cation [CH3OH]+ (g) 856.82 846.50 ± 0.32 kJ/mol 32.04131 ± 12538-91-9*0 Methyloxonium [CH3OH2]+ (g) 588.5 574.3 ± 1.2 kJ/mol 33.04925 ± 17836-08-7*0 Hydroxymethylene HCOH (g, gauche triplet) 219.3 216.4 ± 1.1 kJ/mol 30.02598 ± 19710-56-6*3 Aquacarbon H2OC (g, triplet) 442.7 441.1 ± 1.3 kJ/mol 30.02598 ± 81434-73-3*1 Carbonic acid C(O)(OH)2 (g, cis-trans) -596.87 -606.98 ± 0.83 kJ/mol 62.0248 ± 463-79-6*2 Carbonic acid C(O)(OH)2 (g, trans-trans) -561.64 -570.49 ± 0.97 kJ/mol 62.0248 ± 463-79-6*3 Hydrogen carbonate [HOC(O)O]- (g) -720.5 -726.9 ± 1.4 kJ/mol 61.0174 ± 71-52-3*0 n-Propylium [CH3CH2CH2]+ (g) 856.96 838.68 ± 0.85 kJ/mol 43.0871 ± 19252-52-9*0 Propylidene CH3CH2CH (g, triplet) 349.45 335.76 ± 0.96 kJ/mol 42.0797 ± 6700-78-3*1 Allyl anion [CH2CHCH2]- (g) 134.08 123.07 ± 0.23 kJ/mol 41.0723 ± 1724-46-5*0 Cyclopropyl CH(CH2CH2) (g) 303.4 290.8 ± 1.3 kJ/mol 41.0718 ± 2417-82-5*0 Propylidyne CH3CH2C (g, quartet) 624.6 613.0 ± 1.3 kJ/mol 41.0718 ± 85056-54-8*2 Cycloprop-2-enylium [CH(CHCH)]+ (g) 1080.3 1075.2 ± 1.0 kJ/mol 39.0554 ± 26810-74-2*0 1-Propynylide [CH3CC]- (g) 264.35 260.77 ± 0.70 kJ/mol 39.0565 ± 36147-87-2*0 1-Propen-1-yl-3-ylidene CHCHCH (g, c-c quartet) 656.6 653.2 ± 1.1 kJ/mol 39.0559 ± 84654-90-0*11 sec-Butyl CH3CH2CHCH3 (g) 90.99 66.22 ± 0.87 kJ/mol 57.1143 ± 2348-55-2*0 t-Butyl anion [(CH3)3C]- (g) 76.7 51.9 ± 1.3 kJ/mol 57.1148 ± 65114-21-8*0 Hypochloroperoxoous acid, hydroxy ester HOOOCl (g, syn) 49.2 42.2 ± 1.9 kJ/mol 84.4588 ± 928652-99-7*1 Trioxidanyl HOOO (g, trans) 25.32 23.34 ± 0.13 kJ/mol 49.00614 ± 12500-78-6*1 Oxirene O(CHCH) (g, t-triplet) 387.8 383.6 ± 1.4 kJ/mol 42.0367 ± 157-18-6*11 Oxiranylidene CH2(CO) (g, singlet) 213.5 209.2 ± 1.4 kJ/mol 42.0367 ± 50983-85-2*2 Dioxirane CH2(OO) (g) 9.43 1.76 ± 0.55 kJ/mol 46.0254 ± 157-26-6*0 Hydroxyformyl anion [HOCO]- (g, trans) -313.64 -315.44 ± 0.58 kJ/mol 45.0180 ± 78944-70-4*1 Ethoxyethene CH2CHOCH2CH3 (g, cis-trans) -117.7 -141.5 ± 1.5 kJ/mol 72.1057 ± 109-92-2*2 Oxygen atom O (g, singlet) 436.666 438.523 ± 0.0021 kJ/mol 15.99940 ± 17778-80-2*2 Ethyl formate HC(O)OCH2CH3 (g, syn) -375.3 -394.0 ± 1.2 kJ/mol 74.0785 ± 109-94-4*1 Methoxyoxomethyl CH3OCO (g, anti-staggered) -152.6 -160.7 ± 1.5 kJ/mol 59.0440 ± 16481-04-2*1 Hydroxyde [OH]- (aq) -230.010 ± 0.028 kJ/mol 17.00789 ± 14280-30-9*800 (Formyloxy)methyl HC(O)OCH2 (g, syn) -151.8 -160.0 ± 1.3 kJ/mol 59.0440 ± 3122-12-1*1 Ethylene glycol (CH2OH)2 (g) -369.74 -389.37 ± 0.40 kJ/mol 62.0678 ± 107-21-1*0 Dioxoethyl OCHCO (g) -63.6 -65.0 ± 1.3 kJ/mol 57.0281 ± 702699-38-5*0 Oxalic acid (C(O)OH)2 (g) -721.0 -731.6 ± 1.2 kJ/mol 90.0349 ± 144-62-7*0 (Methylamino)methyl cation [CH3NHCH2]+ (g) 729.27 710.65 ± 0.97 kJ/mol 44.0752 ± *31277-24-4*0 Diazomethyl HCNN (g) 468.17 465.62 ± 0.98 kJ/mol 41.03212 ± 20813-32-5*0 Isocyanoamidogen HNNC (g) 490.3 488.3 ± 1.5 kJ/mol 41.03212 ± 947408-73-3*0 Iodine monobromide IBr (g) 49.719 40.772 ± 0.067 kJ/mol 206.8085 ± 7789-33-5*0 Dichloromethyl CHCl2 (g) 92.20 90.28 ± 0.88 kJ/mol 83.9240 ± 3474-12-2*0 Chloromethyl CH2Cl (g) 118.46 115.39 ± 0.87 kJ/mol 49.4793 ± 6806-86-6*0 Methyl hypochlorite CH3OCl (g) -53.0 -63.0 ± 1.4 kJ/mol 66.4866 ± 593-78-2*0 1,2-Dichloroethane CH2ClCH2Cl (g) -118.59 -130.54 ± 0.54 kJ/mol 98.9586 ± 107-06-2*0 Chlorofluoromethane CH2FCl (g) -256.1 -263.3 ± 1.1 kJ/mol 68.4777 ± 593-70-4*0 Difluoromethyliumyl [CF2]+ (g) 908.20 908.63 ± 0.77 kJ/mol 50.00696 ± 54250-40-7*0 Fluoromethyliumylidene [CF]+ (g) 1122.93 1126.15 ± 0.45 kJ/mol 31.00855 ± 33412-11-2*0 1,2-Difluoroacetylene FCCF (g) 2.86 5.69 ± 0.64 kJ/mol 62.0182 ± 689-99-6*0 Formyl chloride CHClO (g) -179.99 -183.20 ± 0.77 kJ/mol 64.4707 ± 2565-30-2*0 Chloroformyl ClCO (g) -21.92 -20.53 ± 0.49 kJ/mol 63.4628 ± 2602-42-8*0 o-Benzyne anion [C6H4]- (g) 413.73 404.62 ± 0.96 kJ/mol 76.0965 ± 100569-88-8*0 Cycloheptene CH2(CHCHCH2CH2CH2CH2 (g) 28.21 -9.02 ± 0.75 kJ/mol 96.1702 ± 628-92-2*0 Cycloheptatriene CH2(CHCHCHCHCHCH) (g) 207.19 183.77 ± 0.95 kJ/mol 92.1384 ± 544-25-2*0 Fluorobenzene cation [C6H5F]+ (g) 788.89 773.87 ± 0.89 kJ/mol 96.1018 ± 34468-25-2*0 Nitrosobenzene C6H5NO (g) 215.9 199.0 ± 1.2 kJ/mol 107.1100 ± 586-96-9*0 Nitromethyl CH2N(O)O (g) 139.0 129.5 ± 1.2 kJ/mol 60.0321 ± 16787-85-2*0 Nitroxyl ON(O)O (g) 79.41 74.15 ± 0.19 kJ/mol 62.00494 ± 12033-49-7*0 2-Butanone CH3CH2C(O)CH3 (g) -217.00 -238.46 ± 0.79 kJ/mol 72.1057 ± 78-93-3*0 Methylketene CH3CHCO (g) -54.7 -64.2 ± 1.1 kJ/mol 56.0633 ± 6004-44-0*0 Acrolein CH2CHCHO (g, trans) -55.3 -65.7 ± 1.2 kJ/mol 56.0633 ± 107-02-8*1 Formaldoxime CH2NOH (g, trans) 30.5 19.9 ± 1.3 kJ/mol 45.04066 ± 75-17-2*1 Dioxygen anion [O2]- (g) -43.36 -42.72 ± 0.17 kJ/mol 31.99935 ± 11062-77-4*0 1,3-Cyclobutadien-1-yl C(CHCHCH) (g) 653.7 649.2 ± 1.1 kJ/mol 51.0666 ± 127611-22-7*0 Azanylium [NH3]+ (g) 944.272 937.317 ± 0.029 kJ/mol 17.03001 ± 19496-55-0*0 Silylene SiH2 (g, singlet) 272.5 270.8 ± 1.2 kJ/mol 30.10138 ± 13825-90-6*2 Trimethylsiloxide [(CH3)3SiO]- (g) -485.9 -509.8 ± 2.1 kJ/mol 89.1890 ± 41866-81-3*0 1,3-Pentadiyne HCCCCCH3 (g) 417.96 413.74 ± 0.84 kJ/mol 64.0853 ± 4911-55-1*0 1,4-Hexadiyne HCCCH2CCCH3 (g) 424.6 414.6 ± 1.3 kJ/mol 78.1118 ± 10420-91-4*0 Biallenyl CH2CCHCHCCH2 (g, trans) 410.5 398.8 ± 1.5 kJ/mol 78.1118 ± 29776-96-3*1 1,2,3,4-Hexatetraene CH2CCCCHCH3 (g) 429.7 420.0 ± 1.6 kJ/mol 78.1118 ± 177856-38-1*0 (E)-1-Methylallyl CH3CHCHCH2 (g, trans) 154.61 137.30 ± 0.79 kJ/mol 55.0984 ± 17787-86-9*0 Nitrosamine NH2NO (g) 85.00 76.12 ± 0.96 kJ/mol 46.02876 ± 35576-91-1*0 Butadiynyl CCCCH (g) 801.8 808.4 ± 1.0 kJ/mol 49.0507 ± 53561-65-2*0 Peroxynitric acid HOON(O)O (g) -42.6 -52.9 ± 1.3 kJ/mol 79.0123 ± 26404-66-0*0 Nitrosyl chloride ClNO (g) 54.466 52.564 ± 0.068 kJ/mol 65.45884 ± 2696-92-6*0 Hydrogen difluoride (F)(HF) (g, lin) -198.5 -198.4 ± 1.2 kJ/mol 39.004746 ± 12528-21-1*1 Carbon dioxide CO2 (g, triplet) 49.8 50.6 ± 1.1 kJ/mol 44.00950 ± 124-38-9*1 Bicyclo[1.1.0]buta-1,3-diene-2,4-diyl C(CC)C (g) 1047.19 1054.78 ± 0.69 kJ/mol 48.0428 ± 12184-80-4*0 Methane cation [CH4]+ (g) 1150.673 1144.290 ± 0.062 kJ/mol 16.04191 ± 20741-88-2*0 Oxygen atom O (g, triplet) 246.844 249.229 ± 0.0021 kJ/mol 15.99940 ± 17778-80-2*1 Oxoniumyl ion [H2O]+ (g) 978.470 975.567 ± 0.028 kJ/mol 18.01473 ± 56583-62-1*0 Ethanium [C2H7]+ (g) 874.32 858.08 ± 0.62 kJ/mol 31.0764 ± 24669-33-8*0 Acetylene cation [HCCH]+ (g) 1328.85 1328.18 ± 0.13 kJ/mol 26.0367 ± 25641-79-6*0 1-Ethylium-1-ylidene [CH3C]+ (g) 1329.6 1328.2 ± 1.2 kJ/mol 27.0447 ± 78949-84-5*0 Ethylidyne anion [CH3C]- (g) 457.0 452.9 ± 1.2 kJ/mol 27.0458 ± 117768-70-4*0 Chloryl hydride HCl(O)O (g) 197.7 191.2 ± 1.6 kJ/mol 68.4594 ± 174365-32-3*0 Bifluoride [(HF)(F)]- (g, singlet) -481.8 -480.6 ± 1.9 kJ/mol 39.005295 ± *18130-74-0*2 Isocyanomethyl CH2NC (g) 363.8 361.3 ± 1.2 kJ/mol 40.0440 ± 70971-59-4*0 Methyloxoniumylidene [CH3O]+ (g) 1062.71 1054.89 ± 0.75 kJ/mol 31.03337 ± 58157-09-8*0 Hydroxymethylene HCOH (g, cis) 131.10 127.36 ± 0.29 kJ/mol 30.02598 ± 19710-56-6*2 1H-Triazirin-1-yl N(NN) (g) 585.9 584.2 ± 2.0 kJ/mol 42.02022 ± 912535-95-6*0 Hydroxymethylene cation [HCOH]+ (g, trans) 971.9 968.2 ± 1.4 kJ/mol 30.02543 ± 135395-06-1*1 Chlorine difluoride FClF (g) -47.0 -48.2 ± 1.4 kJ/mol 73.44951 ± 24801-48-7*0 Isoformyl COH (g) 217.26 217.64 ± 0.69 kJ/mol 29.01804 ± 71080-92-7*0 Trioxidanylium [HOOO]+ (g, trans) 1070.4 1064.1 ± 1.4 kJ/mol 49.00559 ± 74241-49-9*1 1-Methylethylidene CH3CCH3 (g, triplet) 326.8 313.6 ± 1.2 kJ/mol 42.0797 ± 40852-89-9*1 Allylium [CH2CHCH2]+ (g) 964.58 953.02 ± 0.42 kJ/mol 41.0713 ± 1724-44-3*0 1-Propenyl CH3CHCH (g, trans) 280.36 269.53 ± 0.81 kJ/mol 41.0718 ± 6067-68-1*1 Cyclopropynyl CH(CC) (g) 710.97 715.09 ± 0.85 kJ/mol 37.0400 ± 119178-99-3*0 Hydrogen fluoride HF (aq, 30 H2O) -321.10 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*820 n-Butyl anion [CH3CH2CH2CH2]- (g) 105.9 81.0 ± 1.3 kJ/mol 57.1148 ± 79431-01-9*0 sec-Butyl anion [CH3CH2CHCH3]- (g) 103.1 78.6 ± 1.3 kJ/mol 57.1148 ± 79431-00-8*0 1-Hydroxyethylium [CH3CHOH]+ (g, syn) 610.9 596.4 ± 1.1 kJ/mol 45.0600 ± 18682-96-7*2 Iodine cation I+ (g) 1115.549 1115.149 ± 0.0062 kJ/mol 126.903921 ± 22541-93-1*0 Methoxymethyl CH3OCH2 (g) 12.7 0.2 ± 1.4 kJ/mol 45.0605 ± 16520-04-0*0 Vinyl alcohol cation [CH2CHOH]+ (g, anti) 778.7 767.4 ± 1.3 kJ/mol 44.0520 ± 57239-63-1*2 Oxiranyl CH2(CHO) (g) 172.5 164.2 ± 1.3 kJ/mol 43.0446 ± 31586-84-2*0 Oxiranylium [CH2(CHO)]+ (g, singlet) 901.2 893.2 ± 1.5 kJ/mol 43.0441 ± 57789-76-1*2 Water anion [(H)(OH)]- (g, vdW ?) 31.3 29.8 ± 3.1 kJ/mol 18.01583 ± 12259-30-2*100 C=O=C COC (g, triplet) 653.0 657.3 ± 1.5 kJ/mol 40.0208 ± *12071-23-7*1 C=O=C COC (g, singlet) 721.9 726.2 ± 1.6 kJ/mol 40.0208 ± *12071-23-7*2 Oxoethylidene HCC(O)H (g, singlet) 275.4 272.6 ± 1.7 kJ/mol 42.0367 ± 39920-84-8*2 Ethyl hydroperoxide CH3CH2OOH (g) -143.0 -161.4 ± 1.2 kJ/mol 62.0678 ± 3031-74-1*0 Formic acid cation [HC(O)OH]+ (g, syn) 721.89 714.81 ± 0.26 kJ/mol 46.0248 ± 50614-05-6*1 Dioxidenium [HO2]+ (g) 1110.87 1107.98 ± 0.11 kJ/mol 33.00619 ± 12207-52-2*0 Formyloxidanyl HC(O)O (g, 2B2) -122.09 -125.44 ± 0.56 kJ/mol 45.0174 ± 16499-21-1*2 Diazenyl NNH (g) 252.14 249.22 ± 0.45 kJ/mol 29.02142 ± 36882-13-0*0 (Formyloxy)methyl HC(O)OCH2 (g, anti) -136.2 -144.4 ± 1.3 kJ/mol 59.0440 ± 3122-12-1*2 Carboxymethyl CH2C(O)OH (g, anti) -206.6 -215.9 ± 1.2 kJ/mol 59.0440 ± 2887-46-9*2 Dimethylamide [(CH3)2N]- (g) 122.4 104.0 ± 1.7 kJ/mol 44.0763 ± 34285-60-4*0 Hydrogen chloride HCl (aq, 100 H2O) -165.757 ± 0.025 kJ/mol 36.46064 ± 7647-01-0*828 1,1,2-Trichloroethane CH2ClCHCl2 (g) -136.6 -148.3 ± 1.4 kJ/mol 133.4033 ± 79-00-5*0 1,1,1,2-Tetrachloroethane CH2ClCCl3 (g) -144.5 -153.1 ± 1.3 kJ/mol 167.8481 ± 630-20-6*0 Pentachloroethyl CCl3CCl2 (g) 27.0 25.1 ± 1.3 kJ/mol 201.2849 ± 7094-17-9*0 Iodomethane CH3I (l) -12.17 ± 0.19 kJ/mol 141.93899 ± 74-88-4*590 Difluoromethylene CF2 (g, triplet) 43.30 44.01 ± 0.36 kJ/mol 50.00751 ± 2154-59-8*1 Bromide Br- (aq) -120.59 ± 0.15 kJ/mol 79.90455 ± 24959-67-9*800 Ethyl iodide CH3CH2I (l) -9.41 -39.28 ± 0.49 kJ/mol 155.9656 ± 75-03-6*500 cis-1,2-Difluoroethene CHFCHF (g, cis) -302.90 -309.66 ± 0.90 kJ/mol 64.0341 ± 1630-77-9*0 Fluoroethynyl CCF (g) 445.3 449.8 ± 1.6 kJ/mol 43.0198 ± 22533-50-2*0 Fluoro(fluorooxy)methylene FCOF (g, cis) -134.8 -136.0 ± 1.3 kJ/mol 66.00691 ± 479587-30-9*2 Dibromophosgene CBr2O (g) -97.95 -113.89 ± 0.37 kJ/mol 187.8181 ± 593-95-3*0 Benzene C6H6 (cr,l) 50.72 49.18 ± 0.22 kJ/mol 78.1118 ± 71-43-2*500 o-Benzyne C6H4 (g) 468.12 459.20 ± 0.87 kJ/mol 76.0960 ± 462-80-6*0 m-Benzyne C6H4 (g, singlet) 527.0 518.5 ± 1.2 kJ/mol 76.0960 ± 1828-89-3*2 p-Benzyne C6H4 (g, singlet) 587.0 577.5 ± 1.2 kJ/mol 76.0960 ± 3355-34-8*2 Cycloheptane CH2(CH2CH2CH2CH2CH2C (g) -72.73 -118.22 ± 0.53 kJ/mol 98.1861 ± 291-64-5*0 Benzoyl fluoride C6H5C(O)F (g) -288.0 -304.4 ± 1.5 kJ/mol 124.1124 ± 455-32-3*0 Fluoride F- (aq) -334.50 ± 0.16 kJ/mol 18.99895178 ± 16984-48-8*800 Hydroxyimidogen NOH (g) 217.85 214.97 ± 0.77 kJ/mol 31.01408 ± 35337-59-8*0 Dinitrogen pentoxide O2NONO2 (g) 24.45 14.88 ± 0.35 kJ/mol 108.0105 ± 10102-03-1*0 2-Cyclopropen-1-one OC(CHCH) (g) 163.7 159.8 ± 1.2 kJ/mol 54.0474 ± 2961-80-0*0 Iodide I- (aq) -56.823 ± 0.090 kJ/mol 126.905019 ± 20461-54-5*800 Cyanovinylidene CCHCN (g) 581.7 584.2 ± 1.2 kJ/mol 51.0468 ± 70277-81-5*0 Isocyanovinylidene CCHNC (g) 697.0 700.3 ± 1.4 kJ/mol 51.0468 ± 737788-76-0*0 Cyanoacetylide [CCCN]- (g) 300.3 305.6 ± 1.2 kJ/mol 50.0394 ± 12373-53-4*0 Isocyanoethene CH2CHNC (g, singlet) 285.14 278.64 ± 0.94 kJ/mol 53.0627 ± 14668-82-7*1 1-Cyanoethylidene CH3CCN (g, triplet) 437.5 433.7 ± 1.5 kJ/mol 53.0627 ± 498544-34-6*1 Isocyanomethylene HCNC (g, singlet) 582.3 583.6 ± 1.7 kJ/mol 39.0361 ± 78694-65-2*1 2-Methyl-2-cyclopropen-1-ylidene CH3C(CHC) (g, singlet) 447.9 441.7 ± 1.2 kJ/mol 52.0746 ± 185841-59-2*2 1,2,3-Butatrien-1-ylium [CH2CCCH]+ (g, triplet) 1425.7 1426.0 ± 1.3 kJ/mol 51.0661 ± 81932-79-8*1 1,2,3-Butatrien-1-yl anion [CH2CCCH]- (g) 388.5 386.6 ± 1.3 kJ/mol 51.0672 ± 102275-54-7*0 But-1-en-3-yn-1-ide [HCCCHCH]- (g, cis) 419.7 418.5 ± 1.3 kJ/mol 51.0672 ± 119611-65-3*2 Azide [NNN]- (g) 193.56 190.05 ± 0.91 kJ/mol 42.02077 ± 14343-69-2*0 Propargyl chloride HCCCH2Cl (g) 186.5 180.6 ± 1.3 kJ/mol 74.5086 ± 624-65-7*0 Dioxosilane SiO2 (g) -277.8 -279.1 ± 2.0 kJ/mol 60.08430 ± 7631-86-9*0 Silylene SiH2 (g, triplet) 362.3 360.7 ± 1.2 kJ/mol 30.10138 ± 13825-90-6*1 Benzvalene ((CHCH)(CH(CHCH)CH)) (g) 404.3 386.5 ± 1.2 kJ/mol 78.1118 ± 659-85-8*0 Methylidyne CH (g, doublet) 592.825 596.159 ± 0.097 kJ/mol 13.01864 ± 3315-37-5*1 Hydrogen difluoride cation [FFH]+ (g) 1174.3 1171.3 ± 1.5 kJ/mol 39.004198 ± *126950-54-7*0 Methylammonium [CH3NH3]+ (g) 628.5 609.6 ± 1.4 kJ/mol 32.06453 ± 17000-00-9*0 Aminomethylene CHNH2 (g) 245.9 238.2 ± 1.5 kJ/mol 29.04126 ± 35430-17-2*0 Methylimidogen CH3N (g) 323.1 315.3 ± 1.1 kJ/mol 29.04126 ± 27770-42-9*0 Methyleneamidogen anion [CH2N]- (g) 192.77 189.01 ± 0.91 kJ/mol 28.03387 ± 28892-56-0*0 (Z)-Iminomethyl HCNH (g, cis) 294.50 291.01 ± 0.90 kJ/mol 28.03332 ± 54980-11-9*0 Aminomethylidyne CNH2 (g) 371.9 368.4 ± 1.1 kJ/mol 28.03332 ± 84654-89-7*0 Bifluoride [FFH]- (g, triplet) -68.0 -67.6 ± 1.6 kJ/mol 39.005295 ± *18130-74-0*1 Methyleneoxonium CH2OH2 (g) 152.46 144.14 ± 0.49 kJ/mol 32.04186 ± 177788-17-9*0 Peroxynitrous acid HOONO (g, cis, perp) -0.18 -7.23 ± 0.53 kJ/mol 63.01288 ± 14691-52-2*2 Hydroxymethylene anion [HCOH]- (g, trans) 147.1 144.1 ± 1.6 kJ/mol 30.02653 ± 207914-87-2*1 Hydroxymethyliumylidene [COH]+ (g) 986.02 987.66 ± 0.83 kJ/mol 29.01749 ± 60528-75-8*0 Isoformyl anion [(CO)(H)]- (g, vdW singlet ?) 55.7 58.4 ± 1.8 kJ/mol 29.01859 ± 81747-63-9*1 Peroxyformic acid HC(O)OOH (g, trans) -265.3 -273.2 ± 1.6 kJ/mol 62.0248 ± 107-32-4*2 Hydroxyoxomethoxy HOC(O)O (g) -358.8 -364.9 ± 1.5 kJ/mol 61.0168 ± 38330-85-7*0 Hydroperoxycarbonyl HOOCO (g) -72.9 -76.7 ± 1.7 kJ/mol 61.0168 ± 153751-35-0*0 Chlorine difluoride cation [FClF]+ (g) 998.9 996.7 ± 1.7 kJ/mol 73.44896 ± 32629-46-2*0 Propan-1-yl-3-ylidene CH2CH2CH (g, quartet) 554.3 545.6 ± 1.3 kJ/mol 41.0718 ± *50457-59-5*2 1-Propenylidene CH3CHC (g, singlet) 392.3 387.9 ± 1.1 kJ/mol 40.0639 ± 70277-78-0*1 Oxofluoronium [FO]+ (g) 1342.65 1342.60 ± 0.81 kJ/mol 34.99725 ± 12763-67-6*0 1-Propynyl CH3CC (g) 528.30 526.12 ± 0.70 kJ/mol 39.0559 ± 89342-91-6*0 Cycloprop-2-enyl CH(CHCH) (g) 490.19 485.64 ± 0.58 kJ/mol 39.0559 ± 28933-84-8*0 2-Propen-1-yl-3-ylidene CH2CHC (g) 556.55 554.28 ± 0.89 kJ/mol 39.0559 ± 202744-74-9*0 (E)-Diazene cation [HNNH]+ (g, trans) 1132.49 1125.55 ± 0.64 kJ/mol 30.02881 ± 59952-06-6*0 Cyclopropenylidene C(CHCH) (g) 497.03 496.06 ± 0.46 kJ/mol 38.0480 ± 16165-40-5*0 2-Propynylidyne anion [CCCH]- (g) 540.03 544.80 ± 0.83 kJ/mol 37.0406 ± 82893-67-2*0 t-Butylium [(CH3)3C]+ (g) 733.82 710.54 ± 0.98 kJ/mol 57.1137 ± 14804-25-2*0 (Z)-Diazene HNNH (g, cis) 228.83 221.69 ± 0.63 kJ/mol 30.02936 ± 15626-42-3*0 iso-Butyl (CH3)2CHCH2 (g) 96.97 72.98 ± 0.92 kJ/mol 57.1143 ± 4630-45-9*0 Deuterium atom D (g) 219.804 221.717 ± 0.000 kJ/mol 2.01410177800 ± 16873-17-9*0 Methylcyclopropane CH3CH(CH2CH2) (g) 47.8 24.5 ± 1.3 kJ/mol 56.1063 ± 594-11-6*0 n-Nonane CH3(CH2)7CH3 (g) -176.46 -228.25 ± 0.51 kJ/mol 128.2551 ± 111-84-2*0 Ethanol CH3CH2OH (l) -269.74 -277.51 ± 0.22 kJ/mol 46.0684 ± 64-17-5*500 Peroxychlorous acid HOOClO (g, syn) 96.2 90.2 ± 2.1 kJ/mol 84.4588 ± 177778-92-6*1 Dioxyamidogen HNOO (g, cis) 240.4 233.8 ± 1.8 kJ/mol 47.01348 ± 71843-95-3*2 Trioxidaniumyl [HOOOH]+ (g, t, t) 925.5 916.2 ± 1.5 kJ/mol 50.01353 ± 161321-37-5*2 2-Hydroxyethyl CH2CH2OH (g, staggered-anti) -5.5 -18.0 ± 1.3 kJ/mol 45.0605 ± 4422-54-2*3 Ethoxy CH3CH2O (g) 1.15 -12.28 ± 0.45 kJ/mol 45.0605 ± 2154-50-9*0 Oxygen cation monohydrate [(HOH)O]+ (g) 1153.3 1147.8 ± 3.3 kJ/mol 34.01413 ± *31748-17-1*0 Vinyl alcohol cation [CH2CHOH]+ (g, syn) 786.76 775.53 ± 0.85 kJ/mol 44.0520 ± 57239-63-1*1 Vinoxy cation [CH2CHO]+ (g, triplet) 1020.2 1013.1 ± 1.5 kJ/mol 43.0441 ± *35731-40-9*1 Vinoxide [CH2CHO]- (g) -153.03 -160.09 ± 0.68 kJ/mol 43.0452 ± 35731-40-9*0 2-Hydroxyethenyl CHCHOH (g, trans-cis) 138.01 131.26 ± 0.92 kJ/mol 43.0446 ± 108665-16-3*21 Oxiranyl anion [CH2(CHO)]- (g, singlet) 121.2 113.2 ± 1.5 kJ/mol 43.0452 ± 72722-59-9*2 Acetic acid CH3C(O)OH (aq) -485.16 ± 0.23 kJ/mol 60.0520 ± 64-19-7*800 Ketene CH2CO (g, singlet) -45.35 -48.47 ± 0.11 kJ/mol 42.0367 ± 463-51-4*2 COC vdW (CO)(C) (g, triplet) 589.7 598.5 ± 1.5 kJ/mol 40.0208 ± *12071-24-8*1 Oxirene O(CHCH) (g, c-triplet) 389.9 385.7 ± 1.4 kJ/mol 42.0367 ± 157-18-6*12 Dioxiranylidene C(OO) (g) 190.3 191.1 ± 1.4 kJ/mol 44.00950 ± 138832-57-2*0 Xenon cation Xe+ (g) 1170.354 1170.354 ± 0.0062 kJ/mol 131.2895 ± 24203-25-6*0 Formyloxoniumylidene [HC(O)O]+ (g) 1017.3 1013.9 ± 1.4 kJ/mol 45.0169 ± 54375-27-8*0 Dioxymethylene HCOO (g, trans) 364.0 361.7 ± 2.4 kJ/mol 45.0174 ± 438586-67-5*1 2-Propanol CH3CH(OH)CH3 (g) -249.37 -273.46 ± 0.36 kJ/mol 60.0950 ± 67-63-0*0 Perchloric acid HOCl(O)(O)O (g) 13.2 1.8 ± 1.8 kJ/mol 100.4582 ± 7601-90-3*0 Cyanate [NCO]- (g) -221.19 -221.41 ± 0.53 kJ/mol 42.01739 ± 661-20-1*0 Oxazirinyl C(NO) (g) 450.1 450.9 ± 1.5 kJ/mol 42.01684 ± 228850-18-8*0 Hydrogen fluoride HF (aq, 40 H2O) -321.14 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*821 Propionic acid CH3CH2C(O)OH (g, syn) -436.35 -454.25 ± 0.60 kJ/mol 74.0785 ± 79-09-4*1 2-Methoxy-2-oxoethyl CH2C(O)OCH3 (g, syn) -203.4 -218.6 ± 1.6 kJ/mol 73.0706 ± 54668-31-4*1 (Acetyloxy)methyl CH3C(O)OCH2 (g, syn) -200.6 -214.3 ± 1.6 kJ/mol 73.0706 ± 2887-49-2*1 Chlorine fluoride cation [ClF]+ (g) 1165.52 1165.71 ± 0.49 kJ/mol 54.45055 ± 12432-44-9*0 Dimethylmethyleneammonium [(CH3)2NCH2]+ (g) 696.8 671.7 ± 1.3 kJ/mol 58.1018 ± 28149-27-1*0 Trichloromethide [CCl3]- (g) -124.8 -124.3 ± 2.0 kJ/mol 118.3693 ± 14478-07-0*0 2-Hydrotrioxy cation [OO(H)O]+ (g) 1298.2 1291.5 ± 1.5 kJ/mol 49.00559 ± *74241-49-9*0 Hydroxyaminiumyl [NOH]+ (g) 1161.1 1158.2 ± 1.4 kJ/mol 31.01353 ± 63559-88-6*0 1,1,2,2-Tetrachloroethane CHCl2CHCl2 (g) -146.8 -156.2 ± 1.5 kJ/mol 167.8481 ± 79-34-5*0 1,1,2,2-Tetrachloroethane CHCl2CHCl2 (cr,l) -173.0 -202.0 ± 1.5 kJ/mol 167.8481 ± 79-34-5*500 Pentachloroethane CHCl2CCl3 (cr,l) -202.9 ± 2.5 kJ/mol 202.2928 ± 76-01-7*500 Trichloroethene CHClCCl2 (l) -49.2 ± 1.5 kJ/mol 131.3874 ± 79-01-6*590 Tetrachloroethene CCl2CCl2 (l) -62.9 ± 1.1 kJ/mol 165.8322 ± 127-18-4*500 Oxygen anion monohydrate [(HOH)O]- (g) -239.0 -244.3 ± 3.0 kJ/mol 34.01523 ± 31748-17-1*0 Nitrosodioxaziridine ONN(OO) (g, trans) 413.7 407.6 ± 1.8 kJ/mol 76.01168 ± 132871-99-9*1 Trichloroethene CHClCCl2 (g) -11.3 -14.6 ± 1.5 kJ/mol 131.3874 ± 79-01-6*0 Bromodichloromethane CHCl2Br (g) -37.4 -49.1 ± 1.2 kJ/mol 163.8280 ± 75-27-4*0 Difluorodibromomethane CF2Br2 (g) -364.0 -382.0 ± 1.3 kJ/mol 209.8155 ± 75-61-6*0 Dichlorodibromomethane CCl2Br2 (g) 21.0 5.1 ± 1.3 kJ/mol 242.7241 ± 594-18-3*0 Difluorobromomethane CHF2Br (g) -411.15 -424.34 ± 0.46 kJ/mol 130.9194 ± 1511-62-2*0 2-Hydrotrioxy anion [OO(H)O]- (g) 27.5 21.8 ± 1.4 kJ/mol 49.00669 ± *89965-38-8*0 1,2-Difluoroethane CH2FCH2F (g) -434.42 -447.84 ± 0.81 kJ/mol 66.0500 ± 624-72-6*0 Fluoro(fluorooxy)methylene FCOF (g, trans) -37.5 -39.1 ± 1.4 kJ/mol 66.00691 ± 479587-30-9*1 Dinitrogen tetraoxide O2NNO2 (g) 20.18 10.89 ± 0.14 kJ/mol 92.0111 ± 10544-72-6*0 Chlorine chlorite ClOClO (g, gauche) 168.6 166.2 ± 1.3 kJ/mol 102.9042 ± 105206-44-8*3 Phenylium [C6H5]+ (g, triplet) 1250.8 1237.7 ± 1.6 kJ/mol 77.1034 ± 17333-73-2*1 Hydroxyamidogen HNOH (g, cis) 123.4 116.8 ± 1.7 kJ/mol 32.02202 ± 13940-32-4*2 Hydroxyaminylium [HNOH]+ (g, trans) 1019.5 1012.4 ± 2.5 kJ/mol 32.02147 ± 66097-71-0*1 Chlorobenzene cation [C6H5Cl]+ (g) 942.48 928.56 ± 0.61 kJ/mol 112.5561 ± 55450-32-3*0 Chlorobenzene C6H5Cl (cr,l) 11.24 ± 0.59 kJ/mol 112.5566 ± 108-90-7*500 m-Chlorophenyl C6H4Cl (g) 320.1 309.4 ± 1.6 kJ/mol 111.5487 ± 3474-40-6*0 p-Chlorophenyl C6H4Cl (g) 323.0 312.2 ± 1.6 kJ/mol 111.5487 ± 2396-00-1*0 o-Chlorophenyl C6H4Cl (g) 327.4 316.6 ± 1.6 kJ/mol 111.5487 ± 3474-42-8*0 Bromobenzene cation [C6H5Br]+ (g) 994.8 973.3 ± 1.3 kJ/mol 157.0074 ± 55450-33-4*0 Iodobenzene C6H5I (cr,l) 112.9 113.0 ± 1.1 kJ/mol 204.0084 ± 591-50-4*500 Nitrobenzene C6H5N(O)O (g) 81.6 61.6 ± 1.4 kJ/mol 123.1094 ± 98-95-3*0 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-trans, syn) -53.8 -70.8 ± 1.6 kJ/mol 70.0898 ± 59120-04-6*11 Formaldonitrone CH2NHO (g) 78.4 67.1 ± 1.4 kJ/mol 45.04066 ± 67353-84-8*0 Oxywater (H2O)O (g) 64.8 58.3 ± 1.3 kJ/mol 34.01468 ± 171363-09-0*0 2-Cyclopropen-1-imine (CHCH)CNH (g) 401.8 394.1 ± 1.4 kJ/mol 53.0627 ± 31589-17-0*0 1-Isocyanovinyl CH2CNC (g) 519.1 517.3 ± 1.4 kJ/mol 52.0547 ± 321850-14-0*0 Chlorosyl hydride HClO (g) 141.9 139.0 ± 1.7 kJ/mol 52.46004 ± 71572-35-5*0 Aquachlorine cation [ClOH2]+ (g) 821.8 815.0 ± 1.7 kJ/mol 53.46743 ± 142599-65-3*0 Triiodide [I3]- (aq) -51.44 ± 0.80 kJ/mol 380.713959 ± 14900-04-0*800 Vinylvinylidene CH2CHCHC (g, singlet) 494.1 488.7 ± 1.8 kJ/mol 52.0746 ± 148759-16-4*2 But-1-en-3-yn-1-ide [HCCCHCH]- (g, trans) 422.3 421.0 ± 1.3 kJ/mol 51.0672 ± 119611-65-3*1 Tetrahedryl anion [C(CH(CHCH))]- (g) 634.6 630.8 ± 1.8 kJ/mol 51.0672 ± 138805-00-2*0 Silicon carbide SiC (g) 740.5 745.5 ± 2.4 kJ/mol 40.09620 ± 409-21-2*0 Silicon carbide SiC (cr, alpha-hex) -70.5 -71.5 ± 1.5 kJ/mol 40.09620 ± 409-21-2*501 Tetrafluorosilane SiF4 (g) -1608.90 -1614.41 ± 0.63 kJ/mol 104.07911 ± 7783-61-1*0 Disilane SiH3SiH3 (g) 92.3 76.3 ± 1.4 kJ/mol 62.21864 ± 1590-87-0*0 Dimethylsilanone CH3Si(O)CH3 (g) -237.1 -252.5 ± 2.3 kJ/mol 74.1539 ± 47956-45-6*0 Dimethylsilane CH3SiH2CH3 (g) -66.0 -87.5 ± 1.2 kJ/mol 60.1704 ± 1111-74-6*0 Fluorohydrochlorine cation [FClH]+ (g) 975.3 972.3 ± 2.0 kJ/mol 55.45849 ± 70110-64-4*0 Triprismane ((CHCH)((CHCH)(CHCH) (g) 575.9 556.5 ± 1.3 kJ/mol 78.1118 ± 650-42-0*0 Dihydrogen fluoride anion [HFH]- (g, triplet) 133.2 131.9 ± 1.6 kJ/mol 21.01483 ± 117339-92-1*1 Propargylallene CH2CCHCH2CCH (g, gauche) 431.2 419.6 ± 1.4 kJ/mol 78.1118 ± 33142-15-3*3 Phenanthrene C6H4(C2H2(CC(C4H4))) (g) 231.79 202.98 ± 0.82 kJ/mol 178.2292 ± 85-01-8*0 Diacetylide [CCCCH]- (g) 460.9 465.9 ± 1.0 kJ/mol 49.0513 ± 59012-21-4*0 Chlorohydrofluorine cation [ClFH]+ (g) 1000.8 998.1 ± 2.1 kJ/mol 55.45849 ± 70110-63-3*0 Cyanogen iodide anion [ICN]- (g) 86.5 88.9 ± 8.1 kJ/mol 152.92246 ± 1356929-44-6*0 Ammonium nitrate (NH4)NO3 (cr,l) -350.29 -365.26 ± 0.18 kJ/mol 80.04344 ± 6484-52-2*500 Methanol CH3OH (l) -235.27 -238.61 ± 0.15 kJ/mol 32.04186 ± 67-56-1*500 Formaldehyde cation [CH2O]+ (g) 944.866 941.245 ± 0.097 kJ/mol 30.02543 ± 54288-05-0*0 Peroxychlorite [OClOO]- (g) -34.3 -34.3 ± 2.0 kJ/mol 83.4514 ± 852472-63-0*0 Isoformyl anion [COH]- (g, triplet) 267.4 268.0 ± 1.6 kJ/mol 29.01859 ± 81747-63-9*2 Fluorochlorate [ClF]- (g) -256.03 -255.42 ± 0.81 kJ/mol 54.45165 ± 12272-56-9*0 Bromo hypobromite BrOBr (g) 120.5 104.0 ± 1.2 kJ/mol 175.8074 ± 21308-80-5*0 Trihydrogen dioxide anion [HOHOH]- (g) -487.5 -495.8 ± 1.1 kJ/mol 35.02317 ± 12501-19-8*0 Hydrogen fluoride HF (aq, 22 H2O) -321.06 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*902 Allene cation [CH2CCH2]+ (g) 1132.06 1124.91 ± 0.24 kJ/mol 40.0633 ± 65812-77-3*0 Peroxyimidogen NOO (g) 405.4 403.4 ± 1.9 kJ/mol 46.00554 ± *60488-31-5*0 Ethylene cation [CH2CH2]+ (g) 1075.15 1067.94 ± 0.12 kJ/mol 28.0526 ± 34470-02-5*0 2-Propenylidene CHCHCH2 (g, triplet anti) 417.4 409.8 ± 1.5 kJ/mol 40.0639 ± 19527-08-3*11 2-Propenylidene CHCHCH2 (g, singlet) 452.56 445.43 ± 0.91 kJ/mol 40.0639 ± 19527-08-3*2 Cycloprop-1-enylide [CH2(CHC)]- (g) 373.5 369.1 ± 1.8 kJ/mol 39.0565 ± 80251-77-0*0 Trifluoromethide [CF3]- (g) -637.6 -639.4 ± 1.8 kJ/mol 69.00646 ± 54128-17-5*0 2-Cyclopropen-1-ylium-1-yl [C(CHCH)]+ (g) 1382.0 1380.9 ± 1.1 kJ/mol 38.0474 ± 85398-75-0*0 Propynylidene HCCCH (g, cis singlet) 597.6 599.4 ± 1.3 kJ/mol 38.0480 ± 67152-18-5*3 Propenediylidene CHCHC (g) 796.2 797.3 ± 1.4 kJ/mol 38.0480 ± 60731-11-5*0 n-Butylium [CH3CH2CH2CH2]+ (g) 827.5 803.2 ± 1.2 kJ/mol 57.1137 ± 25453-90-1*0 Diiodide [I2]- (g) -178.13 -180.72 ± 0.48 kJ/mol 253.809489 ± 12190-71-5*0 Oxadiazirene O(NN) (g) 351.7 349.9 ± 1.7 kJ/mol 44.01288 ± 227934-45-4*0 iso-Hexane CH3CH2CH2CH(CH3)2 (g) -134.63 -173.88 ± 0.84 kJ/mol 86.1754 ± 107-83-5*0 NON NON (g) 545.5 542.6 ± 1.7 kJ/mol 44.01288 ± 227934-46-5*0 n-Decane CH3(CH2)8CH3 (g) -192.42 -249.17 ± 0.81 kJ/mol 142.2817 ± 124-18-5*0 Ethyloxoniumyl [CH3CH2OH]+ (g) 783.3 766.3 ± 1.7 kJ/mol 46.0679 ± 34480-55-2*0 1-Hydroxyethylium [CH3CHOH]+ (g) 608.75 594.32 ± 0.39 kJ/mol 45.0600 ± 18682-96-7*0 2-Hydroxyethylium [CH2CH2OH]+ (g, staggered-anti) 1019.1 1006.3 ± 1.5 kJ/mol 45.0600 ± 38607-31-7*3 Ethyloxoniumylidene [CH3CH2O]+ (g) 988.6 974.9 ± 1.5 kJ/mol 45.0600 ± 54387-07-4*0 Acetaldehyde cation [CH3CHO]+ (g) 831.92 821.95 ± 0.26 kJ/mol 44.0520 ± 36505-03-0*0 Azidonitrene NNNN (g, Cs) 692.1 687.5 ± 1.4 kJ/mol 56.02696 ± 147363-92-6*2 Oxiranylium [CH2(CHO)]+ (g, triplet) 1233.5 1226.4 ± 1.7 kJ/mol 43.0441 ± 57789-76-1*1 Oxiranyl anion [CH2(CHO)]- (g, triplet) 140.9 134.9 ± 1.6 kJ/mol 43.0452 ± 72722-59-9*1 Iodine I (g, 2P3/2) 107.157 106.757 ± 0.0021 kJ/mol 126.904470 ± 14362-44-8*1 Ketenyl anion [HCCO]- (g) -48.47 -47.54 ± 0.82 kJ/mol 41.0293 ± 64066-01-9*0 Dicarbon monoxide anion [CCO]- (g) 154.18 157.82 ± 0.83 kJ/mol 40.0213 ± 87191-88-6*0 Dicarbon monoxide CCO (g, singlet) 440.22 444.55 ± 0.83 kJ/mol 40.0208 ± 12071-23-7*2 COC vdW (CO)(C) (g, singlet) 726.3 734.6 ± 1.7 kJ/mol 40.0208 ± *12071-24-8*2 C=O-C cation [COC]+ (g, doublet) 1585.8 1592.9 ± 2.7 kJ/mol 40.0203 ± 83483-98-1*1 C=O=C anion [COC]- (g) 550.3 554.1 ± 1.7 kJ/mol 40.0213 ± *87191-88-6*0 Oxirene cation [O(CHCH)]+ (g) 1106.3 1102.6 ± 1.9 kJ/mol 42.0361 ± 94664-69-4*0 Ethynol HCCOH (g, gauche triplet) 400.0 397.6 ± 1.9 kJ/mol 42.0367 ± 32038-79-2*11 Oxoethylidene HCC(O)H (g, anti-triplet) 265.9 262.6 ± 1.6 kJ/mol 42.0367 ± 39920-84-8*11 Vinylidene CCH2 (g, triplet) 610.41 610.11 ± 0.50 kJ/mol 26.0373 ± 2143-69-3*1 Hydroperoxymethyl CH2OOH (g) 72.9 65.6 ± 1.4 kJ/mol 47.0333 ± 74087-87-9*0 Formic acid cation [HC(O)OH]+ (g, anti) 723.8 716.7 ± 1.2 kJ/mol 46.0248 ± 50614-05-6*2 Formic acid anion [HC(O)OH]- (g, syn) -256.2 -262.4 ± 1.8 kJ/mol 46.0259 ± 19066-82-1*1 Dioxymethylidyne COO (g) 299.8 301.1 ± 1.7 kJ/mol 44.00950 ± 791121-04-5*0 Isocyanic acid cation [HNCO]+ (g) 1002.98 1000.31 ± 0.49 kJ/mol 43.02423 ± 444010-28-0*0 Fulminic acid cation [HCNO]+ (g) 1215.6 1212.9 ± 1.3 kJ/mol 43.02423 ± 114766-65-3*0 Acetic acid CH3C(O)OH (aq, 1500 H2O) -484.89 ± 0.23 kJ/mol 60.0520 ± 64-19-7*840 Triiodide [I3]- (g) -254.3 -257.5 ± 3.4 kJ/mol 380.713959 ± 14900-04-0*0 Dibromide [Br2]- (g) -199.2 -213.3 ± 2.0 kJ/mol 159.8085 ± 12595-70-9*0 Acetyl chloride CH3C(O)Cl (l) -272.13 ± 0.33 kJ/mol 78.4973 ± 75-36-5*500 Methyl formate HC(O)OCH3 (l) -388.50 ± 0.86 kJ/mol 60.0520 ± 107-31-3*500 Ethyl formate HC(O)OCH2CH3 (g, anti) -355.5 -374.6 ± 1.4 kJ/mol 74.0785 ± 109-94-4*2 Diazenide [NNH]- (g, vdW) 175.6 176.7 ± 1.6 kJ/mol 29.02197 ± 71004-29-0*100 2-Methoxy-2-oxoethyl CH2C(O)OCH3 (g, anti) -173.3 -188.5 ± 1.8 kJ/mol 73.0706 ± 54668-31-4*2 (Acetyloxy)methyl CH3C(O)OCH2 (g, anti) -174.3 -187.3 ± 1.7 kJ/mol 73.0706 ± 2887-49-2*2 Hydroxyoxoethenyl HOCCO (g) 24.3 22.5 ± 1.8 kJ/mol 57.0281 ± 702699-39-6*0 Nitronium [ONO]+ (g) 961.80 958.24 ± 0.20 kJ/mol 46.00499 ± 14522-82-8*0 Isocyanomethylidyne CNC (g) 672.2 676.5 ± 1.7 kJ/mol 38.0281 ± 53590-27-5*0 Cyanomethylidyne CCN (g) 684.7 689.1 ± 2.1 kJ/mol 38.0281 ± 4120-02-9*0 Tetrachloromethane CCl4 (l) -108.69 -128.11 ± 0.43 kJ/mol 153.8215 ± 56-23-5*500 Chloride Cl- (aq) -166.992 ± 0.023 kJ/mol 35.45325 ± 16887-00-6*800 Dichloromethylene anion [CCl2]- (g) 75.63 77.91 ± 0.77 kJ/mol 82.9166 ± 118364-73-1*0 Dichloromethylium [CHCl2]+ (g) 894.7 891.5 ± 1.2 kJ/mol 83.9235 ± 56932-33-3*0 Dichloromethide [CHCl2]- (g) -40.9 -42.6 ± 2.0 kJ/mol 83.9246 ± 32180-91-9*0 Chloromethylium [CH2Cl]+ (g) 962.4 958.5 ± 1.2 kJ/mol 49.4787 ± 59000-00-9*0 Chloromethylene CHCl (g, triplet) 346.15 346.45 ± 0.89 kJ/mol 48.4713 ± 2108-20-5*1 1,2-Dichloroethane CH2ClCH2Cl (cr,l) -165.75 ± 0.56 kJ/mol 98.9586 ± 107-06-2*500 1,1-Dichloroethane CH3CHCl2 (cr,l) -163.87 ± 0.48 kJ/mol 98.9586 ± 75-34-3*500 Chlorosyl chloride ClClO (g) 134.5 133.2 ± 1.4 kJ/mol 86.9048 ± 86825-56-1*0 Hydrogen difluoride (F)(HF) (g, perp) -187.4 -187.1 ± 1.6 kJ/mol 39.004746 ± 12528-21-1*2 Pentachloroethane CHCl2CCl3 (g) -151.3 -157.2 ± 1.9 kJ/mol 202.2928 ± 76-01-7*0 Carbide [C2]- (g) 504.74 511.32 ± 0.39 kJ/mol 24.0219 ± 12595-78-7*0 Chloroform CHCl3 (l) -133.47 ± 0.51 kJ/mol 119.3767 ± 67-66-3*590 Chlorodibromomethane CHClBr2 (g) 18.8 0.0 ± 3.4 kJ/mol 208.2793 ± 124-48-1*0 Tribromofluoromethane CBr3F (g) -105.5 -129.5 ± 1.5 kJ/mol 270.7211 ± 353-54-8*0 Tribromochloromethane CBr3Cl (g) 77.6 54.8 ± 1.5 kJ/mol 287.1754 ± 594-15-0*0 Dioxygenyl ion monohydrate [(H2O)(O2)]+ (g) 828.5 821.4 ± 2.1 kJ/mol 50.01353 ± 11083-61-7*0 Ammonia NH3 (aq, undissoc) -80.885 ± 0.053 kJ/mol 17.03056 ± 7664-41-7*1000 Bromochloromethane CH2ClBr (g) -28.1 -42.2 ± 1.3 kJ/mol 129.3833 ± 74-97-5*0 Fluoromethylene CHF (g) 148.39 148.67 ± 0.43 kJ/mol 32.01704 ± 13453-52-6*0 Iminomethide [HCNH]- (g, trans) 237.3 233.5 ± 1.8 kJ/mol 28.03387 ± 90623-30-6*1 Ethyl iodide CH3CH2I (g) 8.70 -7.18 ± 0.49 kJ/mol 155.9656 ± 75-03-6*0 1,2-Dibromoacetylene BrCCBr (g) 333.5 322.6 ± 2.3 kJ/mol 183.8294 ± 624-61-3*0 Fluoro(fluorooxy)methylene FCOF (g, vdW triplet) -97.5 -95.6 ± 1.6 kJ/mol 66.00691 ± 479587-30-9*101 Fluorooxomethylium [FCO]+ (g) 724.9 724.6 ± 1.2 kJ/mol 47.00795 ± 38264-00-5*0 Fluoroisoformyl COF (g) -37.8 -32.4 ± 1.6 kJ/mol 47.00850 ± *1871-24-5*0 Benzene cation [C6H6]+ (g) 992.51 976.05 ± 0.22 kJ/mol 78.1113 ± 34504-50-2*0 2-Cyclopropyn-1-ylidene C(CC) (g, triplet) 898.9 905.8 ± 1.6 kJ/mol 36.0321 ± 102508-15-6*1 2-Cyclopropyn-1-ylium-1-yl [C(CC)]+ (g) 1944.8 1952.7 ± 1.9 kJ/mol 36.0316 ± 154702-35-9*0 Fluoronium [HFH]+ (g) 774.10 771.16 ± 0.88 kJ/mol 21.01373 ± 12206-67-6*0 1-Chloro ozone ClOOO (g, cis vdW) 226.3 223.1 ± 2.3 kJ/mol 83.4509 ± 141801-66-3*101 m-Chlorophenide [C6H4Cl]- (g) 172.3 162.1 ± 1.6 kJ/mol 111.5492 ± 77748-34-6*0 p-Chlorophenide [C6H4Cl]- (g) 179.3 168.9 ± 1.5 kJ/mol 111.5492 ± 77748-42-6*0 o-Chlorophenide [C6H4Cl]- (g) 158.4 148.4 ± 1.6 kJ/mol 111.5492 ± 72863-53-7*0 Fluoronium [(HF)(H)]+ (g, triplet) 1490.1 1491.5 ± 1.9 kJ/mol 21.01373 ± 12206-67-6*1 2-Oxopropylium [CH3C(O)CH2]+ (g) 784.5 770.7 ± 1.8 kJ/mol 57.0707 ± 54088-52-7*0 2,3-Butanedione CH3C(O)C(O)CH3 (g) -310.77 -327.33 ± 0.65 kJ/mol 86.0892 ± 431-03-8*0 1,2-Dibromoethane CH2BrCH2Br (g) -11.6 -38.4 ± 1.2 kJ/mol 187.8612 ± 106-93-4*0 Oxochloronium [ClO]+ (g) 1150.86 1150.70 ± 0.66 kJ/mol 51.45155 ± 12301-79-0*0 2-Propynal CHCCHO (g) 135.5 133.3 ± 1.4 kJ/mol 54.0474 ± 624-67-9*0 Fluorosyl fluoride FFO (g, triplet) 192.8 193.3 ± 2.3 kJ/mol 53.99621 ± 86825-57-2*1 1,3-Butadiyne-1,4-diyl CCCC (g) 1052.40 1062.37 ± 0.66 kJ/mol 48.0428 ± 78015-07-3*0 Dihydrogen fluoride anion [(F)(HH)]- (g, singlet) -257.0 -260.1 ± 1.9 kJ/mol 21.01483 ± *117339-92-1*2 1-Isocyanoethylidene CH3CNC (g, triplet) 545.5 540.7 ± 1.6 kJ/mol 53.0627 ± *498544-34-6*1 3-Imino-2-propen-1-ylidene CHCHCNH (g, anti-triplet) 571.8 566.8 ± 1.6 kJ/mol 53.0627 ± 916765-05-4*21 2-(Methylidyneamino)ethenyl CHCHNCH (g, anti-triplet) 634.5 629.8 ± 1.6 kJ/mol 53.0627 ± 123416-74-0*21 Azete (CHCHCH)N (g) 469.4 461.3 ± 1.5 kJ/mol 53.0627 ± 287-24-1*0 2-Cyanovinylium [CHCHCN]+ (g, anti triplet) 1517.2 1515.1 ± 1.8 kJ/mol 52.0542 ± 85398-67-0*21 2-Cyanovinyl anion [CHCHCN]- (g, syn) 263.8 260.8 ± 1.8 kJ/mol 52.0553 ± *85398-67-0*2 2-Isocyanovinyl CHCHNC (g, anti) 543.2 541.2 ± 1.7 kJ/mol 52.0547 ± 225798-14-1*1 3-Oxo-1,2-propadienylidene CCCO (g) 330.0 335.9 ± 2.0 kJ/mol 52.0315 ± 11127-17-6*0 Dioxygen O2 (g, singlet) 94.410 94.383 ± 0.000 kJ/mol 31.99880 ± 7782-44-7*2 Vinylacetylene cation [CH2CHCCH]+ (g) 1221.5 1215.0 ± 1.1 kJ/mol 52.0740 ± 59699-48-8*0 Bicyclo[1.1.0]but-2-ylium-1-yl [CH2(CCH)CH]+ (g) 1286.8 1277.8 ± 1.5 kJ/mol 52.0740 ± *78525-41-4*0 Hydroxydioxonitrate [HON(O)O]- (g) -193.2 -197.6 ± 2.4 kJ/mol 63.01343 ± 58875-09-5*0 Nitrous acid HONO (g, cis) -71.38 -77.73 ± 0.26 kJ/mol 47.01348 ± 7782-77-6*2 2-Cyclopropen-1-ylidenemethyl CHC(CHCH) (g) 635.6 632.5 ± 1.3 kJ/mol 51.0666 ± 127611-25-0*0 5-Methyl-1,3-cyclopentadiene CH3CH(CHCHCHCH) (g) 133.6 110.7 ± 1.4 kJ/mol 80.1277 ± 96-38-8*0 Disilicon Si2 (g) 587.8 591.7 ± 1.7 kJ/mol 56.17100 ± 12597-35-2*0 Disiloxane SiH3OSiH3 (g) -330.0 -347.0 ± 2.3 kJ/mol 78.21804 ± 13597-73-4*0 Tetramethylsilane Si(CH3)4 (g) -184.1 -214.6 ± 2.1 kJ/mol 88.2236 ± 75-76-3*0 Trimethylsilyl Si(CH3)3 (g) 47.8 25.8 ± 2.2 kJ/mol 73.1891 ± 16571-41-8*0 Hydroxysilyl anion [SiH2OH]- (g, syn) -219.2 -227.2 ± 2.1 kJ/mol 47.10927 ± 78450-47-2*1 1,2,3,5-Hexatetraene CH2CCCHCHCH2 (g, trans) 391.6 379.8 ± 1.6 kJ/mol 78.1118 ± 60239-33-0*1 1,2,3-Pentatriene CH2CCCHCH3 (g) 302.4 290.1 ± 1.4 kJ/mol 66.1011 ± 62018-46-6*0 Hydrogen fluoride HF (aq, 50 H2O) -321.16 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*822 Azanylium [NH2]+ (g) 1266.57 1264.49 ± 0.11 kJ/mol 16.02207 ± 15194-15-7*0 Peroxyimidogen anion [NOO]- (g) 165.0 162.7 ± 1.7 kJ/mol 46.00609 ± 60488-31-5*0 Chlorine difluoride anion [FClF]- (g) -504.9 -505.8 ± 1.9 kJ/mol 73.45005 ± 16892-05-0*0 Ethylene anion [CH2CH2]- (g, anti ?) 232.0 224.4 ± 2.0 kJ/mol 28.0537 ± 34527-91-8*1 Trioxidanylium [HOOO]+ (g, cis) 1085.2 1078.9 ± 1.4 kJ/mol 49.00559 ± 74241-49-9*2 Propynylidene cation [HCCCH]+ (g) 1410.9 1413.4 ± 1.3 kJ/mol 38.0474 ± 75123-91-0*0 Nirous oxide cation [NNO]+ (g) 1329.68 1326.78 ± 0.12 kJ/mol 44.01233 ± 12269-46-4*0 Propynylidene HCCCH (g, trans singlet) 597.4 599.2 ± 1.1 kJ/mol 38.0480 ± 67152-18-5*4 Chlorine difluoride ClFF (g) 22.5 23.8 ± 1.9 kJ/mol 73.44951 ± 91483-78-2*0 Propadienylidenide [CH2CC]- (g) 381.11 382.01 ± 0.41 kJ/mol 38.0485 ± 109292-49-1*0 1,2-Propadien-1-ylium-1-yl [CH2CC]+ (g) 1561.6 1562.8 ± 1.4 kJ/mol 38.0474 ± 110714-00-6*0 (E)-Diazene anion [HNNH]- (g, trans) 265.5 258.7 ± 2.0 kJ/mol 30.02991 ± *59952-06-6*0 Nitrosyl fluoride FNO (g) -85.81 -88.18 ± 0.93 kJ/mol 49.00454 ± 7789-25-5*0 Fluorodioxidenium [FOO]+ (g) 1221.9 1219.5 ± 2.0 kJ/mol 50.99665 ± 139584-95-5*0 2-Butyn-1-yl anion [CH3CCCH2]- (g) 253.4 244.6 ± 1.6 kJ/mol 53.0830 ± 65114-66-1*0 (Z)-Diazene cation [HNNH]+ (g, cis) 1157.0 1150.1 ± 1.3 kJ/mol 30.02881 ± 86631-37-0*0 1-Methylene-2-propenyl CH2CHCCH2 (g) 327.03 317.03 ± 0.94 kJ/mol 53.0825 ± 1204515-52-5*0 Fluorodioxidanide [FOO]- (g) -256.8 -256.1 ± 1.9 kJ/mol 50.99775 ± 23553-17-5*0 n-Pentane CH3CH2CH2CH2CH3 (cr,l) -156.02 -172.99 ± 0.31 kJ/mol 72.1488 ± 109-66-0*500 iso-Pentane CH3CH2CH(CH3)2 (l) -178.39 ± 0.43 kJ/mol 72.1488 ± 78-78-4*500 neo-Pentane C(CH3)4 (l) -189.78 ± 0.39 kJ/mol 72.1488 ± 463-82-1*500 Cyclopentane CH2(CH2CH2CH2CH2) (l) -105.11 ± 0.42 kJ/mol 70.1329 ± 287-92-3*590 Dioxofluorine OFO (g) 524.0 523.2 ± 1.0 kJ/mol 50.99720 ± 175861-93-5*0 n-Hexane CH3CH2CH2CH2CH2CH3 (cr,l) -179.95 -198.60 ± 0.34 kJ/mol 86.1754 ± 110-54-3*500 Cyclohexane CH2(CH2CH2CH2CH2CH2) (l) -156.08 ± 0.29 kJ/mol 84.1595 ± 110-82-7*500 Oxadiazirine anion [O(NN)]- (g) 95.1 95.5 ± 2.9 kJ/mol 44.01343 ± 350586-31-1*0 1,5-Hexadiene CH2CHCH2CH2CHCH2 (g) 108.40 83.81 ± 0.40 kJ/mol 82.1436 ± 592-42-7*0 Ethane cation [CH3CH3]+ (g) 1043.12 1029.11 ± 0.59 kJ/mol 30.0685 ± 34488-65-8*0 Methyleneoxonium cation [CH2OH2]+ (g) 827.7 818.3 ± 1.7 kJ/mol 32.04131 ± *177788-17-9*0 Perchloryloxy OCl(O)(O)O (g) 248.7 241.1 ± 1.8 kJ/mol 99.4503 ± 12133-63-0*0 Perchlorate [OCl(O)(O)O]- (g) -263.2 -272.1 ± 2.2 kJ/mol 99.4508 ± 14797-73-0*0 Hydroxymethanide [CH2OH]- (g) 9.4 2.8 ± 1.7 kJ/mol 31.03447 ± 55830-71-2*0 Nitric acid HON(O)O (cr,l) -179.02 -173.30 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*500 Acetylene anion [HCCH]- (g, trans ?) 356.8 356.7 ± 1.9 kJ/mol 26.0378 ± 50443-89-5*1 Formaldehyde CH2O (g, singlet) -105.376 -109.214 ± 0.096 kJ/mol 30.02598 ± 50-00-0*2 Trioxodinitrate anion [ONN(O)O]- (g, vdW) -104.2 -107.5 ± 1.9 kJ/mol 76.01223 ± 194856-62-7*100 Dicarbon monoxide cation [CCO]+ (g) 1433.9 1439.0 ± 2.1 kJ/mol 40.0203 ± 12287-08-0*0 1-Chloro ozone anion [ClOOO]- (g, perp) -108.9 -111.4 ± 2.2 kJ/mol 83.4514 ± 224299-15-4*3 Azidonitrene NNNN (g, C2h) 660.8 657.6 ± 1.6 kJ/mol 56.02696 ± 147363-92-6*1 C=O-C cation [COC]+ (g, quartet) 1816.8 1820.9 ± 2.0 kJ/mol 40.0203 ± 83483-98-1*2 Oxirene anion [O(CHCH)]- (g) 288.3 285.3 ± 2.2 kJ/mol 42.0372 ± *157-18-6*0 Hydroxymethylene cation [HCOH]+ (g, cis) 986.4 982.8 ± 1.6 kJ/mol 30.02543 ± 135395-06-1*2 Hydroxymethylene cation [HCOH]+ (g, gauche quartet) 1482.1 1479.4 ± 1.9 kJ/mol 30.02543 ± 135395-06-1*3 Oxoethylidene HCC(O)H (g, triplet) 261.1 258.2 ± 1.1 kJ/mol 42.0367 ± 39920-84-8*1 Hydroxymethylene anion [HCOH]- (g, cis) 160.2 157.5 ± 1.7 kJ/mol 30.02653 ± 207914-87-2*2 Hydroxymethylene anion [HCOH]- (g, gauche quartet) 334.0 334.8 ± 2.1 kJ/mol 30.02653 ± 207914-87-2*3 Formic acid anion [HC(O)OH]- (g, anti) -241.2 -247.0 ± 1.9 kJ/mol 46.0259 ± 19066-82-1*2 1-Hydrazinylium-1-yl [NNH2]+ (g) 1143.4 1136.4 ± 1.4 kJ/mol 30.02881 ± 63986-30-1*0 Dioxirane cation [CH2(OO)]+ (g) 1050.8 1043.2 ± 2.0 kJ/mol 46.0248 ± 232262-10-1*0 Dioxymethyl cation [CH2OO]+ (g) 1075.0 1068.6 ± 1.8 kJ/mol 46.0248 ± 232262-11-2*0 Dihydrogen cation [H2]+ (g) 1488.364 1488.480 ± 0.000 kJ/mol 2.01533 ± 12184-90-6*0 Dioxymethylene HCOO (g, cis) 341.8 339.8 ± 3.0 kJ/mol 45.0174 ± 438586-67-5*2 OH-CO vdW complex (OH)(CO) (g, vdW) -80.26 -76.53 ± 0.88 kJ/mol 45.0174 ± *2564-85-4*100 CO-HO vdW complex (CO)(HO) (g, vdW) -77.4 -73.2 ± 1.3 kJ/mol 45.0174 ± *2564-84-3*100 Cyanato cation [NCO]+ (g) 1261.49 1261.99 ± 0.58 kJ/mol 42.01629 ± 17247-99-3*0 Nitrosomethyliumylidene [CNO]+ (g) 1509.8 1510.9 ± 1.7 kJ/mol 42.01629 ± 67197-19-7*0 Fulminate [CNO]- (g) 59.5 59.8 ± 1.7 kJ/mol 42.01739 ± 28269-67-2*0 Acetic acid CH3C(O)OH (l) -484.08 -483.65 ± 0.18 kJ/mol 60.0520 ± 64-19-7*500 Acetic acid CH3C(O)OH (aq, 75 H2O) -484.69 ± 0.23 kJ/mol 60.0520 ± 64-19-7*825 Acetic acid CH3C(O)OH (aq, 700 H2O) -484.88 ± 0.23 kJ/mol 60.0520 ± 64-19-7*835 Aquacarbon H2OC (g, singlet vdW) 467.6 465.5 ± 1.9 kJ/mol 30.02598 ± 81434-73-3*102 Aquacarbon cation [H2OC]+ (g) 1196.1 1193.0 ± 1.7 kJ/mol 30.02543 ± *81434-73-3*0 Hydrogen fluoride HF (aq, 60 H2O) -321.18 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*823 Diazenide [NNH]- (g, triplet) 347.4 344.6 ± 1.6 kJ/mol 29.02197 ± 71004-29-0*1 Glyoxal OCHCHO (g, cis) -189.27 -194.71 ± 0.75 kJ/mol 58.0361 ± 107-22-2*2 Glyoxal OCHCHO (g) -207.71 -213.27 ± 0.61 kJ/mol 58.0361 ± 107-22-2*0 Ethynediol HOCCOH (g) -18.4 -22.2 ± 1.7 kJ/mol 58.0361 ± 16005-17-7*0 1-Chloro ozone anion [ClOOO]- (g, cis) -66.8 -68.7 ± 2.1 kJ/mol 83.4514 ± 224299-15-4*1 Hydrogen fluoride HF (aq, 18 H2O) -321.03 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*901 Dimethylaminylium [(CH3)2N]+ (g) 1040.3 1024.9 ± 1.8 kJ/mol 44.0752 ± 49784-85-2*0 Diisocyanogen CNNC (g) 610.9 613.2 ± 1.7 kJ/mol 52.0349 ± 78800-21-2*0 Hydrogen fluoride HF (aq, 10 H2O) -320.88 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*815 Hydroxylamine NH2OH (g, cis) -15.4 -25.9 ± 1.4 kJ/mol 33.02996 ± 7803-49-8*2 Carbonic acid C(O)(OH)2 (g) -602.73 -612.04 ± 0.75 kJ/mol 62.0248 ± 463-79-6*0 Dichloromethylene CCl2 (g, triplet) 313.63 314.84 ± 0.90 kJ/mol 82.9161 ± 1605-72-7*1 Hydroxylamine cation [NH2OH]+ (g) 857.7 847.9 ± 1.6 kJ/mol 33.02941 ± 62675-55-2*0 Nitric acid HON(O)O (aq, 1000 H2O) -206.32 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*839 Peroxyformic acid HC(O)OOH (g, gauche) -265.2 -273.1 ± 1.5 kJ/mol 62.0248 ± 107-32-4*3 Chloromethyliumyl [CHCl]+ (g) 1207.4 1207.6 ± 2.0 kJ/mol 48.4708 ± 89877-51-0*0 Chloromethylene anion [CHCl]- (g) 203.44 204.26 ± 0.94 kJ/mol 48.4719 ± 75466-08-9*0 Chlorohydroxymethyl CH(Cl)OH (g, anti-gauche) -46.1 -51.7 ± 2.0 kJ/mol 65.4787 ± 147139-06-8*2 Dihydrogen fluoride (H)(HF) (g) -55.9 -54.9 ± 1.6 kJ/mol 21.01428 ± 12592-22-2*1 1,1,2-Trichloroethane CH2ClCHCl2 (l) -188.6 ± 1.4 kJ/mol 133.4033 ± 79-00-5*500 1,1,1,2-Tetrachloroethane CH2ClCCl3 (cr,l) -193.5 ± 1.1 kJ/mol 167.8481 ± 630-20-6*500 Hexachloroethane CCl3CCl3 (cr,l) -216.5 ± 2.8 kJ/mol 236.7376 ± 67-72-1*500 Ethynylene cation [C2]+ (g) 1965.21 1971.79 ± 0.50 kJ/mol 24.0209 ± 12595-79-8*0 3-Chloro-3-methyl-3H-diazirine (NN)CClCH3 (g) 251.4 239.5 ± 2.7 kJ/mol 90.5114 ± 4222-21-3*0 trans-1,2-Dichloroethene CHClCHCl (g, trans) 5.30 -0.12 ± 0.54 kJ/mol 96.9427 ± 156-60-5*0 Hydrogen peroxide cation [H2O2]+ (g, cis) 930.99 924.13 ± 0.98 kJ/mol 34.01413 ± 12325-10-9*2 Trioxidaniumyl [HOOOH]+ (g, c, t) 935.2 926.1 ± 1.5 kJ/mol 50.01353 ± 161321-37-5*3 Trifluoride ion [F3]- (g) -348.8 -349.7 ± 2.6 kJ/mol 56.9957582 ± 25730-99-8*0 Bifluoride [FHF]- (g) -705.21 -709.06 ± 0.36 kJ/mol 39.005295 ± 18130-74-0*0 Dioxaziridinyl cation [N(OO)]+ (g) 1365.9 1363.3 ± 1.7 kJ/mol 46.00499 ± *66252-29-7*0 Aminyliumyl [NH]+ (g) 1659.04 1659.96 ± 0.24 kJ/mol 15.014131 ± 19067-62-0*0 Fluorochlorobromomethane CHFClBr (g) -218.3 -230.9 ± 4.9 kJ/mol 147.3737 ± 593-98-6*0 Nitrogen hydride oxide NH3O (g) 71.2 60.0 ± 1.5 kJ/mol 33.02996 ± 38544-48-8*0 Hydrogen fluoride HF (l) -302.41 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*590 Fluoromethyliumyl [CHF]+ (g) 1116.6 1116.9 ± 1.7 kJ/mol 32.01649 ± 89877-52-1*0 Fluoromethylene anion [CHF]- (g) 95.93 96.47 ± 0.61 kJ/mol 32.01759 ± 71267-42-0*0 Polytetrafluoroethylene CF2CF2 (s) -829.66 ± 0.58 kJ/mol 100.0150 ± 9002-84-0*591 Fluoroniumyl ion [HF]+ (g) 1275.561 1275.793 ± 0.049 kJ/mol 20.005795 ± 12381-92-9*0 Hydrofluorate ion [HF]- (g) -75.6 -75.1 ± 1.8 kJ/mol 20.006892 ± 37249-79-9*0 trans-1,2-Difluoroethene CHFCHF (g, trans) -299.66 -306.10 ± 0.90 kJ/mol 64.0341 ± 1630-78-0*0 Aminooxy cation [NH2O]+ (g) 946.10 938.97 ± 0.79 kJ/mol 32.02147 ± *51375-41-8*0 Fluoroethynide [CCF]- (g) 133.3 137.5 ± 1.5 kJ/mol 43.0204 ± 85905-56-2*0 Iminomethide [HCNH]- (g, cis) 252.6 248.9 ± 1.8 kJ/mol 28.03387 ± 90623-30-6*2 Formyl fluoride cation [CHFO]+ (g) 816.6 813.3 ± 1.3 kJ/mol 48.01589 ± 99766-68-4*0 Formyl fluoride [FCO]- (g) -405.1 -403.7 ± 1.6 kJ/mol 47.00905 ± 62227-71-8*0 Fluoroisoformyl cation [COF]+ (g) 1347.4 1349.5 ± 1.9 kJ/mol 47.00795 ± *1871-25-6*0 Deuterium atom cation D+ (g) 1532.210 1534.123 ± 0.000 kJ/mol 2.01355319809 ± 14464-47-2*0 Chlorine oxide peroxide OClOO (g, vdW) 226.5 227.4 ± 2.5 kJ/mol 83.4509 ± 141801-67-4*100 o-Benzyne cation [C6H4]+ (g) 1392.8 1384.8 ± 1.7 kJ/mol 76.0954 ± 70220-84-7*0 Hydrogen chloride HCl (aq, 2000 H2O) -166.683 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*841 m-Benzyne cation [C6H4]+ (g) 1364.8 1356.2 ± 1.8 kJ/mol 76.0954 ± 121058-08-0*0 p-Benzyne C6H4 (g, triplet) 603.5 594.1 ± 1.2 kJ/mol 76.0960 ± 3355-34-8*1 Benzylium [C6H5CH2]+ (g) 929.47 909.98 ± 0.57 kJ/mol 91.1299 ± 6711-19-9*0 1,3-Cycloheptadiene CH2(CHCHCHCHCH2CH2) (g, c-c) 123.33 94.22 ± 0.78 kJ/mol 94.1543 ± 4054-38-0*1 Tropylium [CH(CHCHCHCHCHCH)]+ (g) 898.1 878.3 ± 1.1 kJ/mol 91.1299 ± 26811-28-9*0 Hydroxyaminylium [HNOH]+ (g, cis) 1048.5 1041.4 ± 2.6 kJ/mol 32.02147 ± 66097-71-0*2 Hydrogen cyanide cation [HCN]+ (g) 1442.51 1442.05 ± 0.20 kJ/mol 27.02483 ± 12601-62-6*0 Hydroxyamidogen anion [HNOH]- (g) 90.7 84.8 ± 2.8 kJ/mol 32.02257 ± *66097-71-0*0 Acetone enol CH3C(OH)CH2 (g, anti) -147.3 -164.6 ± 1.3 kJ/mol 58.0791 ± 29456-04-0*2 Nitrosyl nitrate ON(O2)NO (g, perp) 70.7 62.3 ± 1.8 kJ/mol 92.0111 ± *15969-55-8*0 cis-1,2-Dibromoethene CHBrCHBr (g, cis) 121.2 100.7 ± 2.0 kJ/mol 185.8453 ± 590-11-4*0 1,1-Dibromoethane CH3CHBr2 (g) -5.0 -32.0 ± 1.8 kJ/mol 187.8612 ± 557-91-5*0 Nitrilomethylium [CN]+ (g) 1783.16 1786.64 ± 0.48 kJ/mol 26.01689 ± 12539-57-0*0 Trioxidanyl HOOO (g, cis) 26.30 23.03 ± 0.19 kJ/mol 49.00614 ± 12500-78-6*2 Carbamoyl HNCOH (g, trans-trans) 74.1 67.7 ± 2.7 kJ/mol 44.03272 ± *2858-51-7*1 Formaldiminoxy CH2NO (g) 160.9 153.9 ± 1.5 kJ/mol 44.03272 ± 2683-96-7*0 (Hydroxyimino)methyl HCNOH (g, trans-trans) 250.1 243.9 ± 1.8 kJ/mol 44.03272 ± 206442-92-4*1 Formaldoxime CH2NOH (g, cis) 50.6 39.8 ± 1.8 kJ/mol 45.04066 ± 75-17-2*2 Oxaziridine CH2(NHO) (g) 115.5 103.7 ± 2.1 kJ/mol 45.04066 ± 6827-26-5*0 Cyanoethynyl CCCN (g) 715.7 722.1 ± 1.2 kJ/mol 50.0388 ± 62435-43-2*0 Dihydrooxooxygen cation [(H2O)O]+ (g) 994.8 988.1 ± 1.6 kJ/mol 34.01413 ± 177329-59-8*0 2-Methylene-2H-azirine (CHN)CCH2 (g) 404.2 396.8 ± 1.7 kJ/mol 53.0627 ± 110027-86-6*0 2-Cyanovinylium [CHCHCN]+ (g, syn triplet) 1517.4 1515.2 ± 1.8 kJ/mol 52.0542 ± 85398-67-0*22 2-Cyanovinyl anion [CHCHCN]- (g, anti) 266.1 263.2 ± 1.8 kJ/mol 52.0553 ± *85398-67-0*1 2-Isocyanovinyl CHCHNC (g, syn) 544.6 542.6 ± 1.7 kJ/mol 52.0547 ± 225798-14-1*2 Cyanomethylene HCCN (g, singlet) 529.5 530.6 ± 1.3 kJ/mol 39.0361 ± 2612-62-6*2 Isocyanomethylene HCNC (g, triplet) 586.3 587.3 ± 1.7 kJ/mol 39.0361 ± 78694-65-2*2 Dioxaziridinyl anion [N(OO)]- (g) 295.9 293.4 ± 1.7 kJ/mol 46.00609 ± 66252-31-1*0 3-Methylenecyclopropene cation [CH2C(CHCH)]+ (g) 1181.2 1174.1 ± 1.4 kJ/mol 52.0740 ± 79105-72-9*0 Chlorite [OClO]- (g) -105.51 -107.68 ± 0.37 kJ/mol 67.4520 ± 14998-27-7*0 3-Methylcycloprop-3-en-2-ylium-1-yl [CH3C(CHC)]+ (g) 1283.7 1277.2 ± 1.5 kJ/mol 52.0740 ± *185841-59-2*0 Vinylvinylidene cation [CH2CHCHC]+ (g, trans) 1429.7 1424.0 ± 2.1 kJ/mol 52.0740 ± 161054-20-2*1 1,2-Butadien-1-ylidene CH3CHCC (g, singlet) 511.7 506.2 ± 2.1 kJ/mol 52.0746 ± 119908-55-3*2 Dihydrogen fluoride anion [(HF)(H)]- (g, singlet) -61.9 -60.5 ± 3.2 kJ/mol 21.01483 ± 117339-92-1*2 Hydrogen bromide HBr (aq, 600 H2O) -120.11 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*834 1-Buten-3-ynylium [HCCCHCH]+ (g, trans) 1482.3 1479.7 ± 1.5 kJ/mol 51.0661 ± 84654-85-3*1 Propyne cation [CH3CCH]+ (g) 1192.65 1186.93 ± 0.38 kJ/mol 40.0633 ± 34526-53-9*0 3-Buten-1-ynylium [CH2CHCC]+ (g, triplet) 1572.5 1570.1 ± 1.7 kJ/mol 51.0661 ± 103928-84-3*1 3-Methylene-1-cyclopropen-1-ylium [CH2C(CHC)]+ (g, singlet) 1398.6 1395.3 ± 1.8 kJ/mol 51.0661 ± 117469-28-0*2 3-Methylene-1-cyclopropen-1-yl anion [CH2C(CHC)]- (g) 417.1 413.3 ± 1.9 kJ/mol 51.0672 ± *117469-28-0*0 Cyclobutenyl CH2(CHCHCH) (g) 335.7 323.2 ± 2.2 kJ/mol 53.0825 ± 24669-29-2*0 Butyraldehyde CH3CH2CH2CHO (g) -185.44 -205.81 ± 0.85 kJ/mol 72.1057 ± 123-72-8*0 Chloroallene CH2CCHCl (g) 181.5 174.8 ± 1.6 kJ/mol 74.5086 ± 3223-70-9*0 1-Chloropropyne CH3CCCl (g) 191.3 187.2 ± 1.6 kJ/mol 74.5086 ± 7747-84-4*0 Methylcyclopentane CH3CH(CH2CH2CH2CH2) (g) -68.76 -106.29 ± 0.37 kJ/mol 84.1595 ± 96-37-7*0 Dioxosilane SiO2 (cr,l) -905.58 -910.56 ± 0.89 kJ/mol 60.08430 ± 7631-86-9*500 Dioxosilane SiO2 (vitr) -825.13 -901.06 ± 0.90 kJ/mol 60.08430 ± 7631-86-9*520 Silicon carbide cation [SiC]+ (g) 1602.2 1606.8 ± 2.6 kJ/mol 40.09565 ± 78393-58-5*0 Silanide [SiH3]- (g) 69.0 63.4 ± 1.6 kJ/mol 31.10987 ± 15807-96-2*0 Silyliumyl [SiH2]+ (g) 1155.9 1154.3 ± 1.1 kJ/mol 30.10083 ± 28149-31-7*0 Fluorosyl fluoride anion [FFO]- (g, doublet) -147.1 -146.8 ± 3.0 kJ/mol 53.99675 ± *86825-57-2*2 Hydrogen chloride HCl (aq, 75 H2O) -165.557 ± 0.026 kJ/mol 36.46064 ± 7647-01-0*825 Tetraoxidanyl (HO2)(O2) (g, vdW) 2.0 0.2 ± 3.0 kJ/mol 65.0055 ± 69696-93-1*100 Peroxyimidogen cation [NOO]+ (g) 1409.7 1406.8 ± 1.9 kJ/mol 46.00499 ± *60488-32-6*0 Hydrogen bromide HBr (aq, 5000 H2O) -120.40 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*844 Cyclopropylidene C(CH2CH2) (g, triplet) 544.6 535.8 ± 1.5 kJ/mol 40.0639 ± 2143-70-6*2 Nitrous acid HONO (g) -72.991 -78.647 ± 0.079 kJ/mol 47.01348 ± 7782-77-6*0 (3E)-1,3-Hexadien-5-yne CH2CHCHCHCCH (g, trans) 358.7 346.4 ± 1.9 kJ/mol 78.1118 ± 5222-77-5*0 Divinylacetylene CH2CHCCCHCH2 (g, cis) 361.7 350.3 ± 2.0 kJ/mol 78.1118 ± 821-08-9*2 1,2,4-Cyclohexatriene CH2(CHCHCHCCH) (g) 419.3 403.5 ± 2.0 kJ/mol 78.1118 ± 124869-33-6*0 Pentatetraene CH2CCCCH2 (g) 452.6 448.6 ± 1.6 kJ/mol 64.0853 ± 21986-03-8*0 2-Methyl-2-propanamine (CH3)3CNH2 (g) -87.16 -120.81 ± 0.55 kJ/mol 73.1369 ± 75-64-9*0 Biphenyl C6H5C6H5 (g) 206.9 178.7 ± 1.2 kJ/mol 154.2078 ± 92-52-4*0 Krypton hydride cation [KrH]+ (g) 1106.33 1104.55 ± 0.69 kJ/mol 84.8074 ± 37276-90-7*0 1-Cyclopropene-1,2-diyl CH2(CC) (g) 791.0 790.6 ± 1.5 kJ/mol 38.0480 ± 202744-75-0*0 Oxiranylidene CH2(CO) (g, triplet) 456.1 452.1 ± 2.2 kJ/mol 42.0367 ± 50983-85-2*1 Oxiranylidene cation [CH2(CO)]+ (g) 1165.4 1161.9 ± 2.5 kJ/mol 42.0361 ± *50983-85-2*0 Oxiranylidene anion [CH2(CO)]- (g) 236.4 232.4 ± 2.3 kJ/mol 42.0372 ± *50983-86-3*0 Nirous oxide anion [NNO]- (g) 99.2 96.7 ± 1.9 kJ/mol 44.01343 ± 12274-12-3*0 Methyl hydroperoxide cation [CH3OOH]+ (g) 833.5 819.5 ± 2.2 kJ/mol 48.0407 ± 112465-88-0*0 Methoxyoxoniumylidene [CH3OO]+ (g) 1012.67 1003.83 ± 0.69 kJ/mol 47.0328 ± 86475-50-5*0 Ethylidene anion [CH3CH]- (g) 325.6 318.3 ± 1.8 kJ/mol 28.0537 ± 35829-99-3*0 Cyclopropenyliumdiyl [CH(CC)]+ (g) 1692.1 1695.0 ± 1.9 kJ/mol 37.0395 ± 127476-05-5*0 Chloroniumyl ion [HCl]+ (g) 1137.797 1137.731 ± 0.0051 kJ/mol 36.46009 ± 12258-94-5*0 Cyclopropynyl anion [CH(CC)]- (g) 518.09 521.07 ± 0.88 kJ/mol 37.0406 ± 136861-42-2*0 Dioxirane anion [CH2(OO)]- (g) -173.0 -179.8 ± 3.4 kJ/mol 46.0259 ± *157-26-6*0 Dioxymethyl anion [CH2OO]- (g) 55.0 49.8 ± 1.4 kJ/mol 46.0259 ± 93207-29-5*0 Tetranitrogen cation [NNNN]+ (g) 1392.8 1392.5 ± 1.5 kJ/mol 56.02641 ± 12185-04-5*0 Methylammoniumyl [CH3NH2]+ (g) 865.91 851.98 ± 0.26 kJ/mol 31.05659 ± 34516-31-9*0 Methanol anion [CH3OH]- (g) -89.1 -99.8 ± 2.8 kJ/mol 32.04241 ± 68474-04-4*0 H-CO2 vdW complex (H)(OCO) (g, vdW) -178.3 -177.5 ± 2.1 kJ/mol 45.0174 ± *2564-83-2*100 Methylamine anion [CH3NH2]- (g) 84.2 69.3 ± 2.8 kJ/mol 31.05769 ± 81096-79-9*0 2-Propanol CH3CH(OH)CH3 (cr,l) -306.09 -318.90 ± 0.36 kJ/mol 60.0950 ± 67-63-0*500 1-Propanol CH3CH2CH2OH (cr,l) -290.52 -302.70 ± 0.24 kJ/mol 60.0950 ± 71-23-8*500 Nitrate [ON(O)O]- (aq) -206.64 ± 0.18 kJ/mol 62.00549 ± 14797-55-8*800 Peroxychloric acid HOOCl(O)O (g) 77.5 69.3 ± 2.6 kJ/mol 100.4582 ± 177778-91-5*0 Oxazirinyl cation [C(NO)]+ (g) 1565.5 1566.2 ± 2.3 kJ/mol 42.01629 ± *228850-18-8*0 Oxazirinyl anion [C(NO)]- (g) 448.2 449.0 ± 2.4 kJ/mol 42.01739 ± 28269-68-3*0 Chlorine difluoride cation [ClFF]+ (g) 1346.3 1344.9 ± 2.1 kJ/mol 73.44896 ± 86825-59-4*0 Chlorine difluoride anion [ClFF]- (g) -319.0 -319.0 ± 2.2 kJ/mol 73.45005 ± 91419-41-9*0 1-Methoxyethenol CH2C(OH)OCH3 (g, anti-syn) -284.9 -304.2 ± 1.7 kJ/mol 74.0785 ± 4453-91-2*1 Hydrogen difluoride cation [FFH]+ (g, singlet) 1174.3 1171.3 ± 1.5 kJ/mol 39.004198 ± *126950-54-7*2 Aminomethyl anion [CH2NH2]- (g) 209.4 199.2 ± 2.1 kJ/mol 30.04975 ± 74215-21-7*0 Ethyl formate HC(O)OCH2CH3 (cr,l) -426.1 ± 1.2 kJ/mol 74.0785 ± 109-94-4*500 Ethanium [C2H7]+ (g, classical Cs I) 888.7 870.3 ± 1.8 kJ/mol 31.0764 ± 24669-33-8*2 Triiodine cation [I3]+ (g) 946.1 941.0 ± 4.1 kJ/mol 380.712861 ± 12596-32-6*0 Ethanium [C2H7]+ (g, classical Cs II) 895.1 877.3 ± 1.9 kJ/mol 31.0764 ± 24669-33-8*3 Methylaminilium [CH3NH]+ (g) 1135.0 1125.2 ± 2.1 kJ/mol 30.04865 ± 49784-84-1*0 Propionic acid CH3CH2C(O)OH (g, anti) -415.8 -433.4 ± 1.3 kJ/mol 74.0785 ± 79-09-4*2 Hydrogen fluoride HF (aq, 23 H2O) -321.07 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*903 Dioxodiazoxane ONONO (g, t, c) 109.3 105.3 ± 1.8 kJ/mol 76.01168 ± 122413-35-8*2 Methyliumylamino [CH2NH]+ (g) 1056.1 1049.3 ± 1.8 kJ/mol 29.04071 ± 65130-65-6*0 Cyanogen cation [NCCN]+ (g) 1598.23 1600.45 ± 0.42 kJ/mol 52.0343 ± 37354-81-7*0 Isocyanogen CNCN (g) 412.1 414.4 ± 1.3 kJ/mol 52.0349 ± 83951-85-3*0 Diazoethenylidene NNCC (g) 658.7 661.3 ± 1.8 kJ/mol 52.0349 ± 130760-10-0*0 Ammonium bromide (NH4)Br (cr) -253.35 -269.93 ± 0.16 kJ/mol 97.9425 ± 12124-97-9*510 Isocyanomethyliumylidene [CNC]+ (g) 1615.7 1621.2 ± 1.9 kJ/mol 38.0276 ± 78271-45-1*0 Isocyanomethylidyne anion [CNC]- (g) 479.2 482.9 ± 2.0 kJ/mol 38.0287 ± *53590-27-5*0 Cyanomethyliumylidene [CCN]+ (g) 1719.8 1725.3 ± 2.1 kJ/mol 38.0276 ± 76619-90-4*0 Cyanomethylidyne anion [CCN]- (g) 410.0 413.7 ± 2.2 kJ/mol 38.0287 ± 12373-35-2*0 2,3-didehydro-1H-azirin-1-yl N(CC) (g) 720.3 724.4 ± 2.1 kJ/mol 38.0281 ± 439586-06-8*0 Hydrogen fluoride HF (aq, 55 H2O) -321.18 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*905 Cyanoaminylium [HNCN]+ (g) 1362.8 1359.7 ± 2.4 kJ/mol 41.03157 ± 205443-18-1*0 Cyanoamidogen anion [HNCN]- (g) 69.90 66.89 ± 0.94 kJ/mol 41.03267 ± 67131-47-9*0 Cyanamide NH2CN (g) 140.8 134.5 ± 1.5 kJ/mol 42.04006 ± 420-04-2*0 Diazomethylene CNN (g) 579.3 580.0 ± 3.8 kJ/mol 40.02418 ± 2468-81-7*0 Hydrogen fluoride HF (aq, 37 H2O) -321.13 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*890 Trichloromethylium [CCl3]+ (g) 854.35 852.83 ± 0.93 kJ/mol 118.3683 ± 27130-34-3*0 Azanidylmethyl [CH2NH]- (g) 224.4 217.2 ± 2.2 kJ/mol 29.04181 ± 127593-73-1*0 Dichloromethyliumyl [CCl2]+ (g) 1121.6 1122.6 ± 1.8 kJ/mol 82.9156 ± 55945-51-2*0 Water H2O (l, eq.press.) -285.828 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*589 Triazirine HN(NN) (g) 464.1 457.7 ± 2.6 kJ/mol 43.02816 ± 157-29-9*0 Hydrogen chloride HCl (aq, 74 H2O) -165.546 ± 0.027 kJ/mol 36.46064 ± 7647-01-0*953 2-Propenylidyne anion [CH2CHC]- (g) 464.5 460.7 ± 1.7 kJ/mol 39.0565 ± 120126-89-8*0 Tribromide [Br3]- (g) -287.4 -308.4 ± 1.9 kJ/mol 239.7125 ± 14522-80-6*0 Aminomethyliumyl [CHNH2]+ (g) 1039.5 1031.9 ± 2.3 kJ/mol 29.04071 ± 89302-94-3*0 Fluorosyl fluoride anion [FFO]- (g, quartet) -56.6 -55.7 ± 2.4 kJ/mol 53.99675 ± *86825-57-2*1 2-Propen-3-yliumylidene [CHCHCH]+ (g) 1494.4 1491.4 ± 1.7 kJ/mol 39.0554 ± 84654-86-4*0 2-Chloro ozone anion [ClO(O)O]- (g) -103.3 -105.5 ± 3.2 kJ/mol 83.4514 ± *141801-67-4*0 2-Propenylidene anion [CHCHCH]- (g) 535.8 532.4 ± 1.7 kJ/mol 39.0565 ± 98705-30-7*0 Aminomethylene anion [CHNH2]- (g) 353.0 346.1 ± 2.3 kJ/mol 29.04181 ± 207914-86-1*0 Deuterium hydride cation [HD]+ (g) 1490.498 1490.587 ± 0.000 kJ/mol 3.021493 ± 12181-16-7*0 Methylimidogen anion [CH3N]- (g) 321.1 313.5 ± 1.4 kJ/mol 29.04181 ± 82539-34-2*0 Chlorosyl chloride cation [ClClO]+ (g) 1132.6 1130.8 ± 2.0 kJ/mol 86.9043 ± *86825-56-1*0 Methyleneaminylium [CH2N]+ (g, triplet) 1456.6 1453.0 ± 2.1 kJ/mol 28.03277 ± 53518-13-1*1 Trifluorine cation [F3]+ (g) 1529.5 1527.4 ± 2.3 kJ/mol 56.9946610 ± 64710-22-1*0 Cyclopentene CH2(CH2CHCHCH2) (l) 6.88 ± 0.52 kJ/mol 68.1170 ± 142-29-0*590 Hydrogen fluoride HF (aq, 55.51 H2O) -321.18 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*891 Chloromethane cation [CH3Cl]+ (g) 1014.97 1008.20 ± 0.25 kJ/mol 50.4867 ± 12538-71-5*0 Hydrogen chloride HCl (aq, 2439 H2O) -166.713 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*951 Cyanogen bromide anion [BrCN]- (g) 58.2 54.5 ± 3.8 kJ/mol 105.9220 ± 54092-05-6*0 Bromine atom Br (g, 2P3/2) 117.917 111.855 ± 0.056 kJ/mol 79.90400 ± 10097-32-2*1 Bromofluoromethane CH2FBr (g) -197.4 -211.9 ± 4.9 kJ/mol 112.9290 ± 373-52-4*0 Chlorosyl chloride anion [ClClO]- (g) -185.4 -184.7 ± 2.3 kJ/mol 86.9053 ± *86825-55-0*0 Fluorodibromomethane CHFBr2 (g) -159.7 -179.3 ± 4.9 kJ/mol 191.8250 ± 1868-53-7*0 Difluorochlorobromomethane CF2ClBr (g) -428.9 -439.9 ± 4.9 kJ/mol 165.3642 ± 353-59-3*0 Dichlorofluorobromomethane CCl2FBr (g) -228.3 -238.3 ± 4.9 kJ/mol 181.8185 ± 353-58-2*0 Dibromofluorochloromethane CBr2FCl (g) -169.9 -186.8 ± 4.9 kJ/mol 226.2698 ± 353-55-9*0 Krypton cation Kr+ (g) 1350.756 1350.756 ± 0.000 kJ/mol 83.7995 ± 16915-28-9*0 Chloryl chloride ClCl(O)O (g) 126.1 122.1 ± 1.7 kJ/mol 102.9042 ± 117489-73-3*0 1-Ethylium-1-yl [CH3CH]+ (g) 1182.0 1174.6 ± 3.2 kJ/mol 28.0526 ± 85944-79-2*0 Cyclohexene CH2(CHCHCH2CH2CH2) (g) 27.03 -4.43 ± 0.32 kJ/mol 82.1436 ± 110-83-8*0 Cyclohexene CH2(CHCHCH2CH2CH2) (cr,l) -22.12 -37.99 ± 0.31 kJ/mol 82.1436 ± 110-83-8*500 1,2-Difluoroacetylene cation [FCCF]+ (g) 1081.7 1084.5 ± 1.3 kJ/mol 62.0177 ± 268566-70-7*0 iso-Proplylide [CH3CHCH3]- (g) 128.6 110.2 ± 1.5 kJ/mol 43.0882 ± 25012-80-0*0 Difluorophosgene cation [CF2O]+ (g) 653.45 650.69 ± 0.51 kJ/mol 66.00636 ± 73256-56-1*0 1,2-Propadien-1-yl-3-ylidene anion [CCC]- (g) 622.0 629.4 ± 1.3 kJ/mol 36.0326 ± 109292-47-9*0 Dibromine Br2 (aq) -2.16 ± 0.20 kJ/mol 159.8080 ± 7726-95-6*800 Hypofluorous acid cation [HOF]+ (g) 1142.01 1138.99 ± 0.87 kJ/mol 36.00519 ± 90243-22-4*0 Chloro(chlorooxy)methylene ClCOCl (g, cis) 73.5 73.7 ± 3.6 kJ/mol 98.9155 ± 479587-31-0*2 Aminooxy anion [NH2O]- (g) 47.4 40.5 ± 1.7 kJ/mol 32.02257 ± 51375-41-8*0 Chlorohydroxymethylene ClCOH (g, trans) 13.9 10.9 ± 2.6 kJ/mol 64.4707 ± 147723-60-2*1 (Chlorooxy)methylene HCOCl (g, cis) 119.1 117.8 ± 2.7 kJ/mol 64.4707 ± 331419-80-8*2 Dibromophosgene CBr2O (l) -144.07 ± 0.95 kJ/mol 187.8181 ± 593-95-3*590 Dioxygen O2 (g, triplet) -0.000 -0.000 ± 0.000 kJ/mol 31.99880 ± 7782-44-7*1 2-Chloroethanol ClCH2CH2OH (l) -314.92 ± 0.53 kJ/mol 80.5132 ± 107-07-3*590 2-Bromoethanol BrCH2CH2OH (l) -274.52 ± 0.47 kJ/mol 124.9645 ± 540-51-2*590 Hydrogen fluoride HF (aq, 22.2 H2O) -321.06 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*888 Phenylium [C6H5]+ (g, singlet) 1148.55 1135.77 ± 0.85 kJ/mol 77.1034 ± 17333-73-2*2 2-Cyclopropyn-1-ylidene C(CC) (g, singlet) 993.2 1000.1 ± 2.5 kJ/mol 36.0321 ± 102508-15-6*2 m-Benzyne anion [C6H4]- (g) 444.1 435.2 ± 1.3 kJ/mol 76.0965 ± 249589-31-9*0 p-Benzyne anion [C6H4]- (g) 465.0 455.4 ± 1.3 kJ/mol 76.0965 ± 219298-17-6*0 2-Cyclopropyn-1-yl anion [C(CC)]- (g) 664.8 672.1 ± 1.6 kJ/mol 36.0326 ± 500574-36-7*0 Cycloheptane CH2(CH2CH2CH2CH2CH2C (cr,l) -126.86 -156.75 ± 0.51 kJ/mol 98.1861 ± 291-64-5*500 Aminomethyliumylidene [CNH2]+ (g) 1168.3 1165.1 ± 2.2 kJ/mol 28.03277 ± 86784-42-1*0 Ethylbenzene C6H5CH2CH3 (cr,l) -12.53 ± 0.55 kJ/mol 106.1650 ± 100-41-4*500 Nitrosyl nitrate ONONO2 (g, trans) 48.3 40.4 ± 1.8 kJ/mol 92.0111 ± 15969-55-8*1 Phenylacetylene C6H5CCH (cr,l) 273.4 ± 1.1 kJ/mol 102.1332 ± 536-74-3*500 Hypochlorous acid cation [HOCl]+ (g) 998.92 995.87 ± 0.90 kJ/mol 52.45949 ± 71606-47-8*0 Phenyloxoniumylidene [C6H5O]+ (g) 901.4 887.1 ± 2.2 kJ/mol 93.1028 ± 41071-17-4*0 Anisole C6H5OCH3 (g) -47.81 -72.66 ± 0.79 kJ/mol 108.1378 ± 100-66-3*0 Hydrogen chloride HCl (aq, 150 H2O) -165.979 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*829 Ethylene anion [CH2CH2]- (g, syn ?) 231.5 225.4 ± 2.0 kJ/mol 28.0537 ± 34527-91-8*2 Fluorobenzene C6H5F (cr,l) -151.80 -149.39 ± 0.89 kJ/mol 96.1023 ± 462-06-6*500 Oxobromonium [BrO]+ (g) 1139.4 1131.7 ± 1.5 kJ/mol 95.9029 ± 142315-39-7*0 Bromobenzene C6H5Br (cr,l) 59.9 ± 1.3 kJ/mol 157.0079 ± 108-86-1*500 Acetone CH3C(O)CH3 (cr,l) -245.00 -248.12 ± 0.35 kJ/mol 58.0791 ± 67-64-1*500 1-Hydroxyethanide [CH3CHOH]- (g) -20.3 -33.7 ± 2.2 kJ/mol 45.0610 ± 88032-18-2*0 Propionaldehyde CH3CH2CHO (cr,l) -218.17 -216.71 ± 0.28 kJ/mol 58.0791 ± 123-38-6*500 Ethyl nitrite CH3CH2ONO (g) -81.38 -98.78 ± 0.88 kJ/mol 75.0666 ± 109-95-5*0 Hydrogen bromide HBr (aq, 2570 H2O) -120.34 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*952 trans-1,2-Dibromoethene CHBrCHBr (g, trans) 121.5 101.7 ± 2.0 kJ/mol 185.8453 ± 590-12-5*0 1,1-Dibromoethene CH2CBr2 (g) 125.4 105.1 ± 2.1 kJ/mol 185.8453 ± 593-92-0*0 Norbornadiene (CHCH)(CHCH2CH)(CHCH (g) 267.5 242.0 ± 1.0 kJ/mol 92.1384 ± 121-46-0*0 Monoammonium succinate (CH2C(O)OH)2(NH3) (cr) -1067.96 ± 0.72 kJ/mol 135.1186 ± 38457-08-8*510 Diammonium succinate (CH2COOH)2(NH3)2 (cr) -1182.50 ± 0.48 kJ/mol 152.1492 ± 2226-88-2*510 Hydrooxonitrate [HNO]- (g) 78.62 75.70 ± 0.99 kJ/mol 31.01463 ± 66057-02-1*0 Nitric acid cation [HON(O)O]+ (g) 1029.13 1020.07 ± 0.69 kJ/mol 63.01233 ± 272109-63-4*0 Hydroxyimidogen anion [NOH]- (g) 229.8 227.4 ± 2.2 kJ/mol 31.01463 ± *35337-59-8*0 Acrolein CH2CHCHO (g, cis) -46.5 -56.8 ± 1.4 kJ/mol 56.0633 ± 107-02-8*2 Ethenedione OCCO (g) 14.9 17.3 ± 2.2 kJ/mol 56.0202 ± 4363-38-6*0 Ethenedione cation [OCCO]+ (g) 909.7 912.8 ± 2.3 kJ/mol 56.0197 ± 12541-99-0*0 Hydrogen chloride HCl (aq, 1500 H2O) -166.637 ± 0.025 kJ/mol 36.46064 ± 7647-01-0*840 Fluorine atom cation F+ (g) 1758.306 1760.604 ± 0.048 kJ/mol 18.99785462 ± 14701-13-4*0 Hypobromous acid cation [HOBr]+ (g) 975.01 964.39 ± 0.53 kJ/mol 96.9108 ± 154804-02-1*0 Dinitrogen pentoxide O2NONO2 (cr,l) -41.02 ± 0.42 kJ/mol 108.0105 ± 10102-03-1*500 3-Imino-2-propen-1-ylidene CHCHCNH (g, anti-singlet) 532.9 527.9 ± 1.8 kJ/mol 53.0627 ± 916765-05-4*12 3-Imino-2-propen-1-ylidene CHCHCNH (g, syn-triplet) 572.5 567.3 ± 2.0 kJ/mol 53.0627 ± 916765-05-4*22 2-(Methylidyneamino)ethenyl CHCHNCH (g, syn-singlet) 604.5 599.6 ± 1.9 kJ/mol 53.0627 ± 123416-74-0*12 2-(Methylidyneamino)ethenyl CHCHNCH (g, syn-triplet) 637.1 632.3 ± 2.0 kJ/mol 53.0627 ± 123416-74-0*22 Hypochlorous acid anion [HOCl]- (g) 198.7 198.4 ± 2.5 kJ/mol 52.46059 ± 34524-94-2*0 1-Cyanovinylium [CH2CCN]+ (g) 1318.3 1317.1 ± 2.2 kJ/mol 52.0542 ± 85399-09-3*0 Hydrogen bromide HBr (aq, 3000 H2O) -120.35 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*842 Diazenium [NH2NH]+ (g) 968.64 957.38 ± 0.73 kJ/mol 31.03675 ± 37369-93-0*0 (Methylmethylene)oxonium [CH3OCH2]+ (g) 685.6 671.3 ± 2.3 kJ/mol 45.0600 ± 23653-97-6*0 Methoxymethyl anion [CH3OCH2]- (g) 23.0 10.0 ± 2.3 kJ/mol 45.0610 ± 61192-30-1*0 Cyclopropylide [CH(CH2CH2)]- (g) 264.7 251.9 ± 1.8 kJ/mol 41.0723 ± 1724-45-4*0 Acetaldehyde anion [CH3CHO]- (g) -62.2 -72.5 ± 2.2 kJ/mol 44.0531 ± 60427-04-5*0 Tetraoxygen O4 (g, C2h singlet) 145.8 142.2 ± 3.8 kJ/mol 63.9976 ± 12596-83-7*22 Butatriene cation [CH2CCCH2]+ (g) 1210.3 1204.4 ± 1.3 kJ/mol 52.0740 ± 65513-85-1*0 3-Methanetetrayl-1,2-cyclopropanediylidene CC(CC) (g) 1154.8 1164.2 ± 1.9 kJ/mol 48.0428 ± 78015-08-4*0 Bicyclo[1.1.0]but-3-ene CH2(CCH)CH (g) 483.1 474.1 ± 1.6 kJ/mol 52.0746 ± 78525-41-4*0 Vinylvinylidene anion [CH2CHCHC]- (g, trans) 405.7 398.9 ± 2.1 kJ/mol 52.0751 ± 167386-49-4*1 Tetrahedrane cation [CH(CH(CHCH))]+ (g) 1326.6 1318.4 ± 1.8 kJ/mol 52.0740 ± 80826-01-3*0 Bicyclo[1.1.0]but-1(3)-ene CH2(CC)CH2 (g) 578.7 570.3 ± 2.0 kJ/mol 52.0746 ± 58208-49-4*0 Bicyclo[1.1.0]but-1(3)-ene cation [CH2(CC)CH2]+ (g) 1461.5 1453.4 ± 2.1 kJ/mol 52.0740 ± *58208-49-4*0 Hydrogen bromide HBr (aq, 2000 H2O) -120.30 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*841 2-Cyclopropyn-1-ylidenemethyl anion [CC(CC)]- (g) 800.9 809.9 ± 1.7 kJ/mol 48.0433 ± 289679-90-9*0 Imidogen anion [NH]- (g) 322.62 322.67 ± 0.16 kJ/mol 15.015229 ± 23841-33-0*0 1-Buten-3-yn-1-yl HCCCHCH (g, cis) 547.7 544.9 ± 1.2 kJ/mol 51.0666 ± 2810-61-9*2 Hypofluorous acid anion [HOF]- (g, quartet) 155.3 154.7 ± 2.6 kJ/mol 36.00629 ± 36505-07-4*2 Trioxidanylium [HOOO]+ (g) 1070.4 1064.2 ± 1.4 kJ/mol 49.00559 ± 74241-49-9*0 1-Hydroxyethenyl CH2COH (g) 121.31 114.93 ± 0.99 kJ/mol 43.0446 ± 81847-74-7*0 3-Buten-1-ynylium [CH2CHCC]+ (g, singlet) 1603.7 1602.7 ± 1.7 kJ/mol 51.0661 ± 103928-84-3*2 3-Methylene-1-cyclopropen-1-ylium [CH2C(CHC)]+ (g, triplet) 1467.5 1463.9 ± 1.6 kJ/mol 51.0661 ± 117469-28-0*1 Triazirinylium ion [N(NN)]+ (g) 1608.9 1606.0 ± 1.6 kJ/mol 42.01967 ± 92220-05-8*0 2-Cyclopropen-1-ylidenemethylium [CHC(CHCH)]+ (g, singlet) 1346.3 1343.7 ± 1.8 kJ/mol 51.0661 ± 112936-36-4*2 1,3-Cyclobutadien-1-ylium [C(CHCHCH)]+ (g, singlet) 1388.2 1383.2 ± 1.9 kJ/mol 51.0661 ± 81913-77-1*2 Cyanic azide NCNNN (g) 502.7 498.3 ± 1.9 kJ/mol 68.03766 ± 764-05-6*0 Phenyl hydroperoxide C6H5OOH (g) 13.0 -6.0 ± 2.1 kJ/mol 110.1106 ± 36112-26-2*0 Methylcyclopentane CH3CH(CH2CH2CH2CH2) (cr,l) -116.71 -138.07 ± 0.36 kJ/mol 84.1595 ± 96-37-7*500 Carbon C (g, triplet) 711.398 716.883 ± 0.045 kJ/mol 12.01070 ± 7440-44-0*1 Dioxosilane SiO2 (cr, quartz) -905.58 -910.56 ± 0.89 kJ/mol 60.08430 ± 7631-86-9*505 Dioxosilane SiO2 (cr, cristobalite) -902.88 -907.74 ± 0.93 kJ/mol 60.08430 ± 7631-86-9*510 Formaldehyde anion [CH2O]- (g) -26.7 -28.3 ± 2.0 kJ/mol 30.02653 ± 27837-46-3*0 Propynylidene HCCCH (g) 543.45 546.45 ± 0.65 kJ/mol 38.0480 ± 67152-18-5*0 Ammonium chloride (NH4)Cl (cr) -311.556 -314.718 ± 0.063 kJ/mol 53.49120 ± 12125-02-9*510 Silylene anion [SiH2]- (g) 168.0 166.4 ± 1.6 kJ/mol 30.10193 ± 54991-62-7*0 Silylidyne SiH (g) 371.45 373.24 ± 0.81 kJ/mol 29.09344 ± 13774-94-2*0 Silyliumylidene [SiH]+ (g) 1134.7 1135.9 ± 1.2 kJ/mol 29.09289 ± 31241-66-4*0 Isocyanomethylium [CH2NC]+ (g) 1272.9 1270.7 ± 2.2 kJ/mol 40.0435 ± 78269-43-9*0 Carbon C (g, singlet) 833.329 838.475 ± 0.045 kJ/mol 12.01070 ± 7440-44-0*2 1-Propenylidene CH3CHC (g, triplet) 630.3 622.8 ± 1.7 kJ/mol 40.0639 ± 70277-78-0*2 Formaldehyde CH2O (g, triplet) 196.015 192.684 ± 0.096 kJ/mol 30.02598 ± 50-00-0*1 Hydrogen chloride HCl (aq, 140 H2O) -165.946 ± 0.026 kJ/mol 36.46064 ± 7647-01-0*907 Ketene cation [CH2CO]+ (g) 882.22 879.05 ± 0.11 kJ/mol 42.0361 ± 64999-16-2*0 Ketene anion [CH2CO]- (g) -16.0 -19.1 ± 2.2 kJ/mol 42.0372 ± 56391-79-8*0 Tribromine Br3 (g) 155.9 134.2 ± 3.2 kJ/mol 239.7120 ± 11094-39-6*0 Fluorosyl fluoride cation [FFO]+ (g, quartet) 1413.1 1413.5 ± 3.5 kJ/mol 53.99566 ± *86825-50-5*1 Ammonium radical NH4 (g) 190.01 179.42 ± 0.43 kJ/mol 18.03850 ± 92075-50-8*0 Dewar benzene (CHCH)(CHCHCHCH) (g) 422.0 405.0 ± 1.8 kJ/mol 78.1118 ± 5649-95-6*0 Acetylene anion [HCCH]- (g, cis ?) 380.1 380.3 ± 1.9 kJ/mol 26.0378 ± 50443-89-5*2 Hydroxyoxoammoniumyl [HONO]+ (g) 984.1 977.7 ± 1.8 kJ/mol 47.01293 ± 80371-48-8*0 Ozone cation [OOO]+ (g) 1352.871 1350.532 ± 0.041 kJ/mol 47.99765 ± 12596-82-6*0 1,2-Hexadien-4-yne CH2CCHCCCH3 (g) 407.3 397.3 ± 1.7 kJ/mol 78.1118 ± 34783-10-3*0 3-Methylene-1-penten-4-yne CH2CHC(CH2)CCH (g, trans) 362.5 349.9 ± 2.2 kJ/mol 78.1118 ± 6929-96-0*1 Oxoethenylium [HCCO]+ (g) 1139.7 1142.4 ± 2.1 kJ/mol 41.0282 ± 92056-63-8*0 3-Methyl-1,4-pentadiyne HCCCH(CH3)CCH (g) 438.9 427.4 ± 1.6 kJ/mol 78.1118 ± 57242-04-3*0 2-Methyl-2-propanamine (CH3)3CNH2 (cr,l) -133.26 -150.74 ± 0.54 kJ/mol 73.1369 ± 75-64-9*500 1,2-Diphenylethane C6H5CH2CH2C6H5 (cr,l) 84.66 50.92 ± 0.76 kJ/mol 182.2610 ± 103-29-7*500 Anthracene C6H4(CH(CC(C4H4)CH)) (g) 255.86 226.84 ± 0.99 kJ/mol 178.2292 ± 120-12-7*0 Anthracene C6H4(CH(CC(C4H4)CH)) (cr,l) 150.49 124.68 ± 0.93 kJ/mol 178.2292 ± 120-12-7*500 Bromine dioxide OBrO (g) 167.1 157.5 ± 3.0 kJ/mol 111.9028 ± 21255-83-4*0 Phenanthrene C6H4(C2H2(CC(C4H4))) (cr,l) 136.13 110.85 ± 0.67 kJ/mol 178.2292 ± 85-01-8*500 Diphenylacetylene C6H5CCC6H5 (cr,l) 334.6 312.7 ± 1.1 kJ/mol 178.2292 ± 501-65-5*500 Hydroperoxyimidogen HOON (g, trans) 103.3 99.8 ± 2.4 kJ/mol 47.01348 ± 877456-58-1*1 Bicyclo[1.1.0]buta-1,1-diene-2,4-diyl C(CCH)C (g) 990.3 993.6 ± 2.0 kJ/mol 49.0507 ± *99417-53-5*0 Benzoic acid C6H5C(O)OH (g) -274.39 -294.19 ± 0.20 kJ/mol 122.1213 ± 65-85-0*0 Hydraziniumyl [NH2NH2]+ (g) 886.6 873.3 ± 2.0 kJ/mol 32.04469 ± 20771-51-1*0 Peroxychlorous acid HOOClO (g, anti) 103.5 97.7 ± 2.5 kJ/mol 84.4588 ± 177778-92-6*2 Carbon dioxide anion [CO2]- (g) -340.6 -340.1 ± 2.2 kJ/mol 44.01005 ± 14485-07-5*0 Cyanoaminyliumyl [NCN]+ (g) 1672.2 1673.6 ± 2.6 kJ/mol 40.02363 ± 316122-03-9*0 Cyanoamidogen anion [NCN]- (g) 211.72 211.65 ± 0.62 kJ/mol 40.02473 ± 189879-95-6*0 Dioxiranylidene cation [C(OO)]+ (g) 1409.1 1410.3 ± 2.6 kJ/mol 44.00895 ± *138832-57-2*0 Ethyl hydroperoxide CH3CH2OOH (cr,l) -210. ± 20. kJ/mol 62.0678 ± 3031-74-1*500 Cyanamide NH2CN (cr) 58.89 ± 0.54 kJ/mol 42.04006 ± 420-04-2*500 Hydrogen fluoride HF (aq, 35 H2O) -321.12 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*904 Diazomethyliumyl [CNN]+ (g) 1641.2 1642.4 ± 4.1 kJ/mol 40.02363 ± 307495-90-5*0 Diazomethylene anion [CNN]- (g) 408.3 408.5 ± 3.8 kJ/mol 40.02473 ± 205498-92-6*0 3H-Diazirin-3-ylidene C(NN) (g) 579.0 579.4 ± 2.7 kJ/mol 40.02418 ± 253308-70-2*0 Dioxiranylidene anion [C(OO)]- (g, ?) -110.2 -109.7 ± 3.0 kJ/mol 44.01005 ± *138832-58-3*0 2-Butynylium [CH3CCCH2]+ (g, singlet) 1085.6 1077.4 ± 1.6 kJ/mol 53.0820 ± 64235-83-2*2 Chloromethyliumylidene [CCl]+ (g) 1282.6 1285.7 ± 2.4 kJ/mol 47.4629 ± 69773-94-0*0 Chloromethylidyne anion [CCl]- (g) 319.7 323.4 ± 2.5 kJ/mol 47.4639 ± 117129-77-8*0 Peroxynitrous acid HOONO (g) -5.80 -11.63 ± 0.36 kJ/mol 63.01288 ± 14691-52-2*0 Formic acid HC(O)OH (cr,l) -431.54 -424.71 ± 0.20 kJ/mol 46.0254 ± 64-18-6*500 Peroxyhypochlorous acid anion [HOOCl]- (g, cis quartet) -32.2 -34.0 ± 2.6 kJ/mol 68.4600 ± *67952-08-3*2 Dioxodiazoxane ONONO (g, c, p) 125.7 120.4 ± 2.1 kJ/mol 76.01168 ± 122413-35-8*3 1,1,1-Trichloroethane CH3CCl3 (l) -177.54 ± 0.49 kJ/mol 133.4033 ± 71-55-6*500 Carbon monoxide CO (g, triplet) 465.578 469.285 ± 0.026 kJ/mol 28.01010 ± 630-08-0*1 Isodiazene anion [NNH2]- (g) 380.9 374.1 ± 2.1 kJ/mol 30.02991 ± *63986-30-1*0 Vinyl chloride CH2CHCl (cr,l) -2.7 1.9 ± 1.9 kJ/mol 62.4979 ± 75-01-4*500 Poly(vinylchloride) CH2CHCl (s, poly) -84.6 -94.0 ± 1.3 kJ/mol 62.4979 ± 9002-86-2*500 (Z)-Diazene anion [HNNH]- (g, cis) 280.0 273.3 ± 2.0 kJ/mol 30.02991 ± *86631-37-0*0 Oxadiazirine cation [O(NN)]+ (g) 1509.9 1507.2 ± 2.4 kJ/mol 44.01233 ± *227934-45-4*0 1,1-Dichloroethene CH2CCl2 (l) -23.80 ± 0.50 kJ/mol 96.9427 ± 75-35-4*500 Dioxymethylidyne cation [COO]+ (g, doublet) 1436.6 1439.0 ± 2.7 kJ/mol 44.00895 ± *791121-04-5*2 Dihydrogen fluoride (HF)(H) (g) -56.5 -54.1 ± 1.7 kJ/mol 21.01428 ± 12592-22-2*2 Peroxyhypochlorite [ClOO]- (g) -252.68 -251.66 ± 0.35 kJ/mol 67.4520 ± 224299-14-3*0 Hydrogen chloride HCl (aq, 3000 H2O) -166.741 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*842 Formyloxidanyl HC(O)O (g) -125.90 -127.26 ± 0.56 kJ/mol 45.0174 ± 16499-21-1*0 Hydrogen difluoride cation [FHF]+ (g, triplet ?) 1241. 1238. ± 17. kJ/mol 39.004198 ± 126950-54-7*1 Dichloromethane CH2Cl2 (l) -122.39 ± 0.54 kJ/mol 84.9320 ± 75-09-2*590 Dioxiranyl HC(OO) (g) 217.3 213.9 ± 2.0 kJ/mol 45.0174 ± 120595-16-6*0 Propane cation [CH3CH2CH3]+ (g) 974.6 954.5 ± 1.7 kJ/mol 44.0951 ± 34479-70-4*0 Hydroxylamine NH2OH (aq, undissoc) -97.1 ± 2.2 kJ/mol 33.02996 ± 7803-49-8*1000 Isoprene CH2C(CH3)CHCH2 (cr,l) 48.82 ± 0.69 kJ/mol 68.1170 ± 78-79-5*500 Azide [NNN]- (aq) 272.64 ± 0.48 kJ/mol 42.02077 ± 14343-69-2*800 Difluoromethylene anion [CF2]- (g) -211.16 -209.98 ± 0.58 kJ/mol 50.00805 ± 66212-40-6*0 Dihydrogen H2 (g, ortho) 1.417 0.019 ± 0.000 kJ/mol 2.01588 ± 1333-74-0*1 Fluoromethylidyne anion [CF]- (g) 200.8 204.3 ± 1.9 kJ/mol 31.00965 ± 33412-12-3*0 Hydrazine NH2NH2 (cr,l) 57.25 50.69 ± 0.18 kJ/mol 32.04524 ± 302-01-2*500 Ethynylene C2 (g, triplet) 827.55 834.26 ± 0.26 kJ/mol 24.0214 ± 12070-15-4*1 Oxygen atom hexacation [O]+6 (g) 42035.28 42037.14 ± 0.12 kJ/mol 15.99611 ± 14158-22-6*0 Trioxidane HOOOH (g) -82.01 -90.61 ± 0.63 kJ/mol 50.01408 ± 14699-99-1*0 2,4,5-Trioxa-1,3-diazabicyclo[1.1.1]pentane N(O3)N (g) 602.5 593.3 ± 2.1 kJ/mol 76.01168 ± 224950-18-9*0 Ethoxyethane CH3CH2OCH2CH3 (cr,l) -265.46 -277.78 ± 0.82 kJ/mol 74.1216 ± 60-29-7*500 Bromosyl bromide BrBrO (g) 179.0 163.2 ± 2.1 kJ/mol 175.8074 ± 68322-97-4*0 Hydrogen fluoride HF (aq, 18.5 H2O) -321.04 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*887 Triiodine I3 (g) 153.4 149.4 ± 3.6 kJ/mol 380.713410 ± 12596-31-5*0 Isocyanogen iodide INC (g) 341.7 342.0 ± 4.9 kJ/mol 152.92191 ± 66004-32-8*0 Difluorovinylidene CCF2 (g) 129.4 130.6 ± 2.2 kJ/mol 62.0182 ± 41895-33-4*0 1,2-Propadien-1-ylium-1-yl-3-ylidene [CCC]+ (g, lin) 1981.5 1989.4 ± 2.2 kJ/mol 36.0316 ± 118090-85-0*1 Fluoroethynylium [CCF]+ (g) 1536.2 1540.9 ± 2.3 kJ/mol 43.0193 ± 268566-69-4*0 n-Heptane CH3(CH2)5CH3 (cr,l) -201.65 -224.11 ± 0.46 kJ/mol 100.2019 ± 142-82-5*500 Bromoacetylene HCCBr (g) 282.2 275.7 ± 1.6 kJ/mol 104.9333 ± 593-61-3*0 n-Octane CH3(CH2)6CH3 (cr,l) -226.49 -249.61 ± 0.55 kJ/mol 114.2285 ± 111-65-9*500 Hydrazoic acid anion [HNNN]- (g, trans) 309.0 302.7 ± 2.3 kJ/mol 43.02871 ± 203264-98-6*1 n-Nonane CH3(CH2)7CH3 (cr,l) -247.83 -274.69 ± 0.50 kJ/mol 128.2551 ± 111-84-2*500 Chloro(chlorooxy)methylene ClCOCl (g, trans singlet) 98. 101. ± 15. kJ/mol 98.9155 ± 479587-31-0*11 Chlorooxomethylium [ClCO]+ (g) 783.7 783.9 ± 2.2 kJ/mol 63.4623 ± 38264-03-8*0 Hydrochlorate ion [HCl]- (g) -25.2 -24.2 ± 2.5 kJ/mol 36.46119 ± 63099-75-2*0 Chlorine hydride oxide HCl(O)(O)O (g) 165.2 155.3 ± 2.5 kJ/mol 84.4588 ± 1027055-52-2*0 2-Iodoethanol ICH2CH2OH (l) -207.2 ± 2.4 kJ/mol 171.9650 ± 624-76-0*590 1,3-Butadiyne cation [HCCCCH]+ (g) 1440.13 1441.99 ± 0.64 kJ/mol 50.0581 ± 55468-55-8*0 Chlorine chlorite ClOClO (g, cis) 177.4 175.0 ± 2.2 kJ/mol 102.9042 ± 105206-44-8*2 Carbon suboxide cation [OCCCO]+ (g) 928.3 931.8 ± 1.3 kJ/mol 68.0304 ± 151528-67-5*0 Carbon suboxide anion [OCCCO]- (g) -131.4 -128.0 ± 2.5 kJ/mol 68.0314 ± 109362-94-9*0 Benzene anion [C6H6]- (g) 209.1 197.2 ± 1.2 kJ/mol 78.1124 ± 34562-85-1*0 Methanol dimer (CH3OH)2 (g) -398.5 -419.1 ± 1.4 kJ/mol 64.0837 ± 42845-44-3*0 Trioxirane O(OO) (g) 272.7 270.1 ± 1.3 kJ/mol 47.99820 ± 153851-84-4*0 Tetraoxodinitrogen cation [O2NNO2]+ (g) 1030.6 1025.2 ± 2.6 kJ/mol 92.0105 ± 64476-91-1*0 n-Decane CH3(CH2)8CH3 (cr,l) -273.01 -300.56 ± 0.80 kJ/mol 142.2817 ± 124-18-5*500 Cyclopropenylidene anion [C(CHCH)]- (g) 544.2 543.8 ± 2.2 kJ/mol 38.0485 ± *16165-40-5*0 o-Benzyne C6H4 (g, triplet) 625.6 616.3 ± 1.1 kJ/mol 76.0960 ± 462-80-6*1 Trioxidanyl HOOO (g) 25.32 23.23 ± 0.13 kJ/mol 49.00614 ± 12500-78-6*0 1,5-Hexadiene CH2CHCH2CH2CHCH2 (cr,l) 54.44 ± 0.32 kJ/mol 82.1436 ± 592-42-7*500 Methylamine CH3NH2 (l) -45.25 ± 0.45 kJ/mol 31.05714 ± 74-89-5*500 m-Benzyne C6H4 (g) 527.0 518.5 ± 1.2 kJ/mol 76.0960 ± 1828-89-3*0 1-Methyl-1-ethylium-1-yl [CH3CCH3]+ (g) 1058.1 1045.6 ± 1.9 kJ/mol 42.0792 ± 121408-19-3*0 p-Benzyne cation [C6H4]+ (g) 1424.9 1417.7 ± 2.1 kJ/mol 76.0954 ± 121058-09-1*0 p-Benzyne C6H4 (g) 587.0 577.6 ± 1.2 kJ/mol 76.0960 ± 3355-34-8*0 Difluorodioxidanium [FOOF]+ (g, trans) 1283.7 1279.1 ± 2.3 kJ/mol 69.99506 ± *7783-44-0*1 Toluene C6H5CH3 (l) 19.82 12.07 ± 0.33 kJ/mol 92.1384 ± 108-88-3*500 Oxygen difluoride anion [FOF]- (g) -202.0 -201.9 ± 2.7 kJ/mol 53.99675 ± *7783-41-7*0 Cyclopentadienylium [CH(CHCHCHCH)]+ (g) 1087.73 1074.87 ± 0.92 kJ/mol 65.0927 ± 29661-18-5*0 Cycloheptene CH2(CHCHCH2CH2CH2CH2 (cr,l) -25.5 -46.6 ± 1.7 kJ/mol 96.1702 ± 628-92-2*500 NON cation [NON]+ (g) 1443.9 1444.1 ± 2.8 kJ/mol 44.01233 ± *227934-46-5*0 Cycloheptatriene CH2(CHCHCHCHCHCH) (cr,l) 153.83 145.05 ± 0.97 kJ/mol 92.1384 ± 544-25-2*500 Phenylethene C6H5CHCH2 (cr,l) 104.31 ± 0.48 kJ/mol 104.1491 ± 100-42-5*500 Tetrazete N(NNN) (g, D2h) 764.9 759.0 ± 1.8 kJ/mol 56.02696 ± 42851-09-2*0 Phenol C6H5OH (cr,l) -146.50 -162.35 ± 0.65 kJ/mol 94.1112 ± 108-95-2*500 Cycloprop-2-enylide [CH(CHCH)]- (g) 514.8 511.8 ± 2.2 kJ/mol 39.0565 ± 20829-57-6*0 Dihydrogen cation [H2]+ (g, ortho) 1489.060 1488.480 ± 0.000 kJ/mol 2.01533 ± 12184-90-6*1 Anisole C6H5OCH3 (l) -119.58 ± 0.77 kJ/mol 108.1378 ± 100-66-3*500 Hydrogen difluoride cation [(HF)(F)]+ (g, triplet) 1267.6 1265.4 ± 4.1 kJ/mol 39.004198 ± *126950-54-7*1 Benzaldehyde C6H5C(O)H (cr,l) -85.7 -86.8 ± 1.1 kJ/mol 106.1219 ± 100-52-7*500 Isopropylidene anion [CH3CCH3]- (g) 282.4 268.2 ± 2.0 kJ/mol 42.0803 ± *121408-19-3*0 2-Propen-3-ylium-1-ylidyne [CHCHC]+ (g) 1791.1 1792.7 ± 2.6 kJ/mol 38.0474 ± 161054-36-0*0 Fluorobenzene anion [C6H5F]- (g) -18.3 -30.2 ± 1.8 kJ/mol 96.1029 ± 34561-55-2*0 m-Chlorophenylium [C6H4Cl]+ (g) 1160.1 1150.0 ± 2.6 kJ/mol 111.5481 ± 127983-85-1*0 p-Chlorophenylium [C6H4Cl]+ (g) 1147.4 1137.9 ± 2.5 kJ/mol 111.5481 ± 42766-43-8*0 o-Chlorophenylium [C6H4Cl]+ (g) 1166.7 1157.4 ± 2.5 kJ/mol 111.5481 ± 137963-70-3*0 1,3-Propanediyl CH2CH2CH2 (g, triplet) 320.0 308.2 ± 1.6 kJ/mol 42.0797 ± 32458-33-6*1 Hydronium radical (H2O)(H) (g, vdW) -18.5 -21.6 ± 2.1 kJ/mol 19.02322 ± 12168-76-2*100 Iodobenzene cation [C6H5I]+ (g) 1022.82 1007.38 ± 0.99 kJ/mol 204.0078 ± 38406-85-8*0 Nitrobenzene C6H5N(O)O (cr,l) 6.6 ± 1.4 kJ/mol 123.1094 ± 98-95-3*500 Hydrogen chloride HCl (aq, 200 H2O) -166.105 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*830 Nitromethane CH3N(O)O (cr,l) -114.15 -112.97 ± 0.43 kJ/mol 61.0401 ± 75-52-5*500 Acetic acid CH3C(O)OH (aq, 1000 H2O) -484.89 ± 0.23 kJ/mol 60.0520 ± 64-19-7*839 2,2,4-Trimethylpentane (CH3)2CHCH2C(CH3)3 (cr,l) -224.4 -258.9 ± 1.5 kJ/mol 114.2285 ± 540-84-1*500 cis-1,2-Diiodoethene CHICHI (g, cis) 217.41 209.13 ± 0.35 kJ/mol 279.8462 ± 590-26-1*0 trans-1,2-Diiodoethene CHICHI (g, trans) 215.07 207.68 ± 0.35 kJ/mol 279.8462 ± 590-27-2*0 Norbornadiene (CHCH)(CHCH2CH)(CHCH (l) 208.7 ± 1.2 kJ/mol 92.1384 ± 121-46-0*500 2,3-Butanedione CH3C(O)C(O)CH3 (cr,l) -365.64 ± 0.53 kJ/mol 86.0892 ± 431-03-8*500 Succinic acid (CH2C(O)OH)2 (g) -802.5 -824.7 ± 3.0 kJ/mol 118.0880 ± 110-15-6*0 Nitric acid trihydrate (HON(O)O)(H2O)3 (cr,l) -1062.10 -1055.25 ± 0.21 kJ/mol 117.0587 ± 13444-83-2*500 1,2-Diiodoethane CH2ICH2I (g) 87.80 73.13 ± 0.90 kJ/mol 281.8621 ± 624-73-7*0 1,2-Diiodoethane CH2ICH2I (cr,l) 9.26 ± 0.60 kJ/mol 281.8621 ± 624-73-7*500 2-Butanone CH3CH2C(O)CH3 (cr,l) -267.89 -273.38 ± 0.79 kJ/mol 72.1057 ± 78-93-3*500 Tetraazatetrahedrane N(N(N)N) (g, Td) 763.7 757.2 ± 1.7 kJ/mol 56.02696 ± 12596-63-3*0 Methyl acetate CH3C(O)OCH3 (l) -445.68 ± 0.60 kJ/mol 74.0785 ± 79-20-9*500 Acrolein CH2CHCHO (cr,l) -95.7 ± 1.4 kJ/mol 56.0633 ± 107-02-8*500 Bicyclo[1.1.0]buta-1,3-dien-4-ylium-2-yl [C(CC)C]+ (g) 2087.7 2097.6 ± 2.3 kJ/mol 48.0423 ± 289679-91-0*0 Bicyclo[1.1.0]buta-1,3-dien-2-yl anion [C(CC)C]- (g) 803.2 810.8 ± 1.8 kJ/mol 48.0433 ± 289679-89-6*0 Ethenedione anion [OCCO]- (g) -149.5 -146.9 ± 2.3 kJ/mol 56.0207 ± 77783-15-4*0 Urea NH2C(O)NH2 (cr,l) -317.78 -333.38 ± 0.12 kJ/mol 60.05534 ± 57-13-6*500 Formamide CH(O)NH2 (cr,l) -251.03 ± 0.29 kJ/mol 45.04066 ± 75-12-7*500 Chloryl ion [OClO]+ (g) 1100.27 1097.61 ± 0.53 kJ/mol 67.4510 ± 25052-55-5*0 Acetamide CH3C(O)NH2 (cr,l) -315.89 ± 0.54 kJ/mol 59.0672 ± 60-35-5*500 Nitrosyl nitrite anion [ONONO]- (g, t, t) -103.0 -106.2 ± 2.8 kJ/mol 76.01223 ± *122413-35-8*1 Nitric acid monohydrate (HON(O)O)(H2O) (cr,l) -479.18 -472.68 ± 0.19 kJ/mol 81.0282 ± 13444-82-1*500 Hydrogen chloride HCl (aq, 1000 H2O) -166.565 ± 0.024 kJ/mol 36.46064 ± 7647-01-0*839 1-Propynylium [CH3CC]+ (g) 1547.0 1545.1 ± 3.1 kJ/mol 39.0554 ± 24858-94-4*0 1-Propenylium [CH3CHCH]+ (g, cis) 1232.0 1221.7 ± 1.7 kJ/mol 41.0713 ± 50457-58-4*2 Acrylonitrile CH2CHCN (g, triplet) 446.4 440.9 ± 1.8 kJ/mol 53.0627 ± 107-13-1*2 Acrylonitrile CH2CHCN (cr,l) 146.27 153.93 ± 0.78 kJ/mol 53.0627 ± 107-13-1*500 1,3-Butadiynyl anion [CCCC]- (g) 677.4 686.5 ± 1.1 kJ/mol 48.0433 ± 59158-53-1*0 Isocyanoethene CH2CHNC (g, triplet) 544.1 539.2 ± 1.8 kJ/mol 53.0627 ± 14668-82-7*2 1-Propenylide [CH3CHCH]- (g, cis) 204.1 192.8 ± 1.8 kJ/mol 41.0723 ± 65887-20-9*2 1-Isocyanoethylidene CH3CNC (g) 527.8 523.1 ± 1.1 kJ/mol 53.0627 ± *498544-34-6*0 Dioxohydrazine ONNO (g, cis) 172.92 171.15 ± 0.14 kJ/mol 60.01228 ± 16824-89-8*2 Acetonitrile CH3CN (cr,l) 40.60 ± 0.22 kJ/mol 41.0520 ± 75-05-8*500 Hydrazoic acid cation [HNNN]+ (g) 1333.68 1328.00 ± 0.83 kJ/mol 43.02761 ± 58852-14-5*0 Dioxohydrazine ONNO (g) 172.92 171.15 ± 0.14 kJ/mol 60.01228 ± 16824-89-8*0 1-Ethenylium-1-yl [CCH2]+ (g) 1507.0 1507.1 ± 2.1 kJ/mol 26.0367 ± 80541-07-7*0 Cyclopropylium [CH(CH2CH2)]+ (g) 1104.6 1093.1 ± 2.4 kJ/mol 41.0713 ± 1724-43-2*0 NON anion [NON]- (g) 495.3 492.8 ± 2.5 kJ/mol 44.01343 ± *227934-47-6*0 Hydrogen fluoride HF (aq, 400 H2O) -321.47 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*832 Tetraoxygen O4 (g, C2h triplet) 104.4 102.4 ± 3.5 kJ/mol 63.9976 ± 12596-83-7*11 Vinylacetylene CH2CHCCH (cr,l) 266.2 ± 2.1 kJ/mol 52.0746 ± 689-97-4*500 Nitric acid HON(O)O (aq, 3 H2O) -197.77 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*805 Perchloryl cation [OCl(O)O]+ (g) 1252.1 1246.4 ± 2.5 kJ/mol 83.4504 ± 23594-88-9*0 Hydrogen peroxide H2O2 (cr,l) -193.163 -187.346 ± 0.079 kJ/mol 34.01468 ± 7722-84-1*500 Isocyanogen iodide anion [INC]- (g) 123.0 125.2 ± 9.3 kJ/mol 152.92246 ± *66004-32-8*0 Vinylvinylidene CH2CHCHC (g, triplet, trans) 690.4 684.3 ± 2.7 kJ/mol 52.0746 ± 148759-16-4*11 Dioxymethylidyne cation [(C)(OO)]+ (g, vdW quartet) 1388.2 1391.7 ± 3.2 kJ/mol 44.00895 ± *791121-04-5*101 Oxygen difluoride cation [FOF]+ (g) 1292.12 1289.59 ± 0.66 kJ/mol 53.99566 ± 39432-25-2*0 Cycloprop-1-enylium [CH2(CHC)]+ (g) 1502.2 1497.9 ± 2.2 kJ/mol 39.0554 ± 117067-78-4*0 2-Cyclopropyn-1-ylidenemethyliumyl [CC(CC)]+ (g) 2239.6 2247.5 ± 2.3 kJ/mol 48.0423 ± 289679-92-1*0 1-Buten-3-ynylium [HCCCHCH]+ (g, cis) 1483.0 1480.4 ± 1.7 kJ/mol 51.0661 ± 84654-85-3*2 Carbon dioxide cation [CO2]+ (g, quartet) 1335.0 1335.5 ± 2.4 kJ/mol 44.00895 ± 12181-61-2*1 Isocyanogen bromide BrNC (g) 341.5 335.8 ± 3.4 kJ/mol 105.9214 ± 65997-98-0*0 Trihydrogen H(HH) (g, 2p 2A2" Ry) 756.931 754.146 ± 0.015 kJ/mol 3.02382 ± 12184-91-7*2 Chlorodioxidanyl ClOO (g) 100.46 99.36 ± 0.35 kJ/mol 67.4515 ± 17376-09-9*0 Propenediylidene anion [CHCHC]- (g) 588.8 590.0 ± 2.3 kJ/mol 38.0485 ± *161054-36-0*0 2-Cyclopropen-1-ylidenemethylium [CHC(CHCH)]+ (g, triplet) 1433.0 1429.2 ± 1.9 kJ/mol 51.0661 ± 112936-36-4*1 Hydroxyoxoammoniumyl [HONO]+ (g, trans) 984.1 977.7 ± 1.8 kJ/mol 47.01293 ± 80371-48-8*1 1,3-Cyclobutadien-1-ylium [C(CHCHCH)]+ (g, triplet) 1472.9 1468.3 ± 1.9 kJ/mol 51.0661 ± 81913-77-1*1 1,3-Cyclobutadien-1-yl anion [C(CHCHCH)]- (g) 616.3 611.8 ± 2.2 kJ/mol 51.0672 ± 154675-37-3*0 Cyclobutenylium [CH2(CHCHCH)]+ (g) 1037.3 1024.2 ± 3.4 kJ/mol 53.0820 ± 12316-90-4*0 2-Hydroxyethenyl CHCHOH (g, cis-cis) 143.9 137.4 ± 1.1 kJ/mol 43.0446 ± 108665-16-3*11 Butyraldehyde CH3CH2CH2CHO (cr,l) -235.97 -239.49 ± 0.84 kJ/mol 72.1057 ± 123-72-8*500 Propionic acid CH3CH2C(O)OH (cr,l) -505.46 -509.60 ± 0.18 kJ/mol 74.0785 ± 79-09-4*500 2-Hydroxyethenyl CHCHOH (g, trans-trans) 147.2 140.9 ± 1.3 kJ/mol 43.0446 ± 108665-16-3*22 2-Hydroxyethenyl CHCHOH (g, cis-trans) 150.6 144.5 ± 1.6 kJ/mol 43.0446 ± 108665-16-3*12 Bromoniumyl [HBr]+ (g) 1098.07 1090.22 ± 0.14 kJ/mol 80.9114 ± 12258-64-9*0 Isocyanogen chloride ClNC (g) 314.5 316.2 ± 2.4 kJ/mol 61.4701 ± 75754-69-7*0 2-Oxo-1-ethylium-1-yl [HCC(O)H]+ (g, anti-quartet) 1289.4 1286.0 ± 2.5 kJ/mol 42.0361 ± 214464-96-7*11 Deuterium hydride HD (g) 0.328 0.319 ± 0.000 kJ/mol 3.022042 ± 13983-20-5*0 Disilicon cation [Si2]+ (g) 1350.2 1353.8 ± 2.8 kJ/mol 56.17045 ± 12597-36-3*0 Dioxosilane cation [SiO2]+ (g) 946.4 946.4 ± 2.8 kJ/mol 60.08375 ± 59458-81-0*0 Silicate [SiO2]- (g) -477.3 -478.1 ± 2.7 kJ/mol 60.08485 ± 53095-83-3*0 Ethylene glycol (CH2OH)2 (cr,l) -448.72 -455.24 ± 0.37 kJ/mol 62.0678 ± 107-21-1*500 Tetraoxidanyl HOOOO (g, cis) 53.0 46.0 ± 5.1 kJ/mol 65.0055 ± 69696-93-1*2 Oxosilicate [SiO]- (g) -105.8 -104.6 ± 2.6 kJ/mol 44.08545 ± 12503-59-2*0 Cyclobutane CH2(CH2CH2CH2) (l) 3.93 ± 0.40 kJ/mol 56.1063 ± 287-23-0*590 Silicon carbide SiC (cr, beta-cubic) -71.9 -72.9 ± 1.5 kJ/mol 40.09620 ± 409-21-2*502 Silane cation [SiH4]+ (g) 1104.9 1097.7 ± 1.7 kJ/mol 32.11671 ± 34826-51-2*0 Hydrogen bromide HBr (aq, 1000 H2O) -120.20 ± 0.15 kJ/mol 80.9119 ± 10035-10-6*839 Silylium [SiH3]+ (g) 989.6 984.0 ± 1.2 kJ/mol 31.10877 ± 41753-67-7*0 Dioxygen difluoride OOF2 (g) 75.1 71.6 ± 3.6 kJ/mol 69.99561 ± 183051-89-0*0 Silylidyne anion [SiH]- (g) 248.6 249.8 ± 1.2 kJ/mol 29.09399 ± 68865-96-3*0 Ammonia anion [NH3]- (g) 94.3 87.2 ± 2.4 kJ/mol 17.03111 ± 37353-00-7*0 Nitric acid HON(O)O (aq, 1 H2O) -186.85 ± 0.18 kJ/mol 63.01288 ± 7697-37-2*801 Peroxyhypochlorous acid cation [HOOCl]+ (g, trans) 1030.3 1024.5 ± 2.5 kJ/mol 68.4589 ± *67952-07-2*1 Oxalic acid (C(O)OH)2 (cr) -819.57 -829.66 ± 0.42 kJ/mol 90.0349 ± 144-62-7*500 Trifluorine F3 (g) 80.6 81.9 ± 2.6 kJ/mol 56.9952096 ± 73455-86-4*0 Dimethylamine (CH3)2NH (l) -43.43 ± 0.37 kJ/mol 45.0837 ± 124-40-3*500 Methylcyclopropane CH3CH(CH2CH2) (l) 1.84 ± 0.59 kJ/mol 56.1063 ± 594-11-6*590 Nitrous acid anion [HONO]- (g, cis) -105.84 -110.46 ± 0.78 kJ/mol 47.01403 ± 256393-36-9*2 2,4-Hexadiyne CH3CCCCCH3 (cr,l) 319.0 312.6 ± 1.0 kJ/mol 78.1118 ± 2809-69-0*500 Iodine monochloride ICl (cr) -36.511 -35.537 ± 0.063 kJ/mol 162.35717 ± 7790-99-0*510 Carbon monoxide anion [CO]- (g) 30.0 33.7 ± 2.3 kJ/mol 28.01065 ± 12539-63-8*0 Trimethylenecyclopropane (C3)(CH2)3 (g) 454.1 441.2 ± 1.8 kJ/mol 78.1118 ± 3227-90-5*0 3,4-Bis(methylene)cyclobutene CH2(CCHCHC)CH2 (g) 355.4 340.8 ± 1.8 kJ/mol 78.1118 ± 5291-90-7*0 Trimethylamine (CH3)3N (l) -48.85 ± 0.87 kJ/mol 59.1103 ± 75-50-3*500 Oxoethylidene anion [HCC(O)H]- (g, anti-gauche) 86.9 83.6 ± 2.1 kJ/mol 42.0372 ± 106543-38-8*1 1-Ethynylcyclobutene HCCC(CHCH2CH2) (g) 402.1 387.8 ± 1.8 kJ/mol 78.1118 ± 813466-25-0*0 3-Methyl-1,2-pentadien-4-yne CH2CC(CH3)CCH (g) 414.5 403.3 ± 2.3 kJ/mol 78.1118 ± 813466-14-7*0 1,3-Pentadiyne HCCCCCH3 (cr,l) 384.9 ± 5.0 kJ/mol 64.0853 ± 4911-55-1*500 Ethylamine CH3CH2NH2 (cr,l) -74.09 ± 0.55 kJ/mol 45.0837 ± 75-04-7*500 1-Propanamine CH3CH2CH2NH2 (cr,l) -93.92 -101.52 ± 0.41 kJ/mol 59.1103 ± 107-10-8*500 2-Propanamine CH3CH(CH3)NH2 (cr,l) -101.67 -112.28 ± 0.52 kJ/mol 59.1103 ± 75-31-0*500 Paraformaldehyde CH2O (cr, paraformaldehyde) -179.2 ± 1.6 kJ/mol 30.02598 ± 30525-89-4*500 Cyanogen anion [NCCN]- (g) 281.5 284.7 ± 2.2 kJ/mol 52.0354 ± 11118-88-0*0 Naphthalene C6H4(C4H4) (cr,l) 94.51 74.92 ± 0.60 kJ/mol 128.1705 ± 91-20-3*500 Diisocyanogen cation [CNNC]+ (g, trans) 1825.3 1828.1 ± 3.2 kJ/mol 52.0343 ± *78800-21-2*1 Biphenyl C6H5C6H5 (cr,l) 121.5 97.1 ± 1.1 kJ/mol 154.2078 ± 92-52-4*500 Biphenyl C6H5C6H5 (cr) 121.5 97.1 ± 1.1 kJ/mol 154.2078 ± 92-52-4*520 Isocyanic fluoride FNC (g) 301.3 303.1 ± 1.7 kJ/mol 45.01584 ± 16992-26-0*0 Benzoic acid C6H5C(O)OH (cr,l) -367.38 -384.80 ± 0.17 kJ/mol 122.1213 ± 65-85-0*500 Helium hydride cation [HeH]+ (g) 1350.148 1348.258 ± 0.000 kJ/mol 5.009993 ± 17009-49-3*0 Xenon hydride cation [XeH]+ (g) 1038.04 1036.26 ± 0.89 kJ/mol 132.2974 ± 37276-91-8*0 Carbon suboxide OCCCO (cr,l) -131.7 -116.6 ± 1.3 kJ/mol 68.0309 ± 504-64-3*500 Peroxyformate [HC(O)OO]- (g, anti) -320.6 -326.5 ± 1.8 kJ/mol 61.0174 ± 104960-05-6*2 Tribromide [Br3]- (aq) -129.17 ± 0.25 kJ/mol 239.7125 ± 14522-80-6*800 Peroxyformyl HC(O)OO (g, anti) -90.4 -95.8 ± 1.8 kJ/mol 61.0168 ± 56240-83-6*2 Oxygen atom tetracation [O]+4 (g) 17719.207 17721.064 ± 0.025 kJ/mol 15.99721 ± 14127-65-2*0 Methylaminyliumyl [CH3N]+ (g, doublet ?) 1388.3 1381.9 ± 2.3 kJ/mol 29.04071 ± 56924-51-7*1 Hydrazoic acid HNNN (aq) 272.64 ± 0.48 kJ/mol 43.02816 ± 7782-79-8*800 m-Benzyne C6H4 (g, triplet) 614.6 605.2 ± 1.4 kJ/mol 76.0960 ± 1828-89-3*1 1-Propen-1-yl-3-ylidene CHCHCH (g, quartet) 656.6 653.2 ± 1.1 kJ/mol 39.0559 ± 84654-90-0*1 Oxygen atom heptacation [O]+7 (g) 113369.48 113371.34 ± 0.12 kJ/mol 15.99556 ± 14274-89-6*0 Hydrogen iodide HI (aq) -56.823 ± 0.090 kJ/mol 127.912410 ± 10034-85-2*800 1-Hydroxyethyl CH3CHOH (g) -42.79 -56.22 ± 0.57 kJ/mol 45.0605 ± 2348-46-1*0 Tetraoxodinitrate anion [O2NNO2]- (g) -184.6 -187.8 ± 2.4 kJ/mol 92.0116 ± 63552-02-3*0 Formylimidogen HC(O)N (g, singlet) 245.3 242.0 ± 3.3 kJ/mol 43.02478 ± 42288-92-6*2 Tropylide [CH(CHCHCHCHCHCH)]- (g) 261.1 244.7 ± 2.0 kJ/mol 91.1310 ± 34464-18-1*0 Hydrogen fluoride HF (aq, 45 H2O) -321.15 ± 0.16 kJ/mol 20.006343 ± 7664-39-3*906 sec-Butylium [CH3CH2CHCH3]+ (g) 792.9 769.3 ± 1.7 kJ/mol 57.1137 ± 16548-59-7*0 Water H2O (cr) -286.298 -292.739 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*510 Nitrosyl nitrate ONONO2 (g, cis-perp) 60.4 52.5 ± 1.9 kJ/mol 92.0111 ± 15969-55-8*2 mu-Oxodichlorine cation [ClOCl]+ (g) 1130.44 1126.84 ± 0.63 kJ/mol 86.9043 ± 51174-96-0*0 Tetrabromomethane CBr4 (cr, monoclinic) 49.3 ± 1.4 kJ/mol 331.6267 ± 558-13-4*500 Tetraiodomethane CI4 (cr, monoclinic) 392.5 ± 8.0 kJ/mol 519.62858 ± 507-25-5*500 Hydrazoic acid anion [HNNN]- (g, cis) 316.2 310.1 ± 2.3 kJ/mol 43.02871 ± 203264-98-6*2 mu-Oxodichlorate anion [ClOCl]- (g) -147.8 -147.7 ± 2.5 kJ/mol 86.9053 ± 365546-30-1*0 Bromomethane CH3Br (l) -55.93 -58.94 ± 0.27 kJ/mol 94.9385 ± 74-83-9*590 Trioxirane cation [O(OO)]+ (g) 1444.5 1442.3 ± 2.2 kJ/mol 47.99765 ± *153851-84-4*0 Hydrazine monohydrate (NH2NH2)(H2O) (l) -242.66 ± 0.19 kJ/mol 50.06052 ± 7803-57-8*500 Water H2O (g, para) -238.929 -241.832 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*2 Difluorodioxidanium [FOOF]+ (g, cis) 1287.2 1283.0 ± 2.3 kJ/mol 69.99506 ± *7783-44-0*2 Acetic acid CH3C(O)OH (aq, 500 H2O) -484.87 ± 0.23 kJ/mol 60.0520 ± 64-19-7*833 Acetic acid CH3C(O)OH (aq, 10000 H2O) -484.92 ± 0.23 kJ/mol 60.0520 ± 64-19-7*850 Fluorine atom F (g, 2P3/2) 77.258 79.043 ± 0.047 kJ/mol 18.99840320 ± 14762-94-8*1 Nitrogen atom N (g, quartet) 470.579 472.442 ± 0.024 kJ/mol 14.006740 ± 17778-88-0*1 Hydrazoic acid HNNN (aq, undissoc) 257.65 ± 0.48 kJ/mol 43.02816 ± 7782-79-8*1000 Hydroxymethylene HCOH (g) 112.68 108.92 ± 0.27 kJ/mol 30.02598 ± 19710-56-6*0 Iodine monochloride ICl (l) -24.048 ± 0.063 kJ/mol 162.35717 ± 7790-99-0*590 Cyclopentadiene CH2(CHCHCHCH) (l) 109.91 ± 0.59 kJ/mol 66.1011 ± 542-92-7*590 Succinic acid (CH2C(O)OH)2 (cr,l) -918.56 -940.28 ± 0.13 kJ/mol 118.0880 ± 110-15-6*500 Methyl acetate CH3C(O)OCH3 (g) -394.22 -413.09 ± 0.60 kJ/mol 74.0785 ± 79-20-9*0 Methylene CH2 (g) 391.060 391.617 ± 0.097 kJ/mol 14.02658 ± 2465-56-7*0 Peroxyhypochlorous acid anion [HOOCl]- (g, trans quartet) -2.9 -3.3 ± 2.6 kJ/mol 68.4600 ± *67952-08-3*1 1,2-Dibromoethane CH2BrCH2Br (cr,l) -80.2 ± 1.2 kJ/mol 187.8612 ± 106-93-4*500 2-Chloro ozone ClO(O)O (g) 266.0 265.4 ± 3.7 kJ/mol 83.4509 ± *141801-66-3*0 Dioxofluorine anion [OFO]- (g) 155.9 154.4 ± 2.5 kJ/mol 50.99775 ± *175861-93-5*0 Trihydrogen cation [H3]+ (g, para) 1110.304 1106.697 ± 0.013 kJ/mol 3.02327 ± 28132-48-1*2 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-cis, syn) -43.1 -59.6 ± 2.3 kJ/mol 70.0898 ± 59120-04-6*21 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-trans) -53.8 -69.1 ± 1.6 kJ/mol 70.0898 ± 59120-04-6*1 Diatomic bromine cation [Br2]+ (g) 1060.32 1045.37 ± 0.23 kJ/mol 159.8075 ± 12595-71-0*0 Acrolein CH2CHCHO (g) -55.3 -65.4 ± 1.2 kJ/mol 56.0633 ± 107-02-8*0 1,2-Propadien-1-one cation [CH2CCO]+ (g) 1012.6 1011.2 ± 1.9 kJ/mol 54.0468 ± 87612-93-9*0 1-Methylethenylium [CH3CCH2]+ (g) 995.3 986.1 ± 2.0 kJ/mol 41.0713 ± 50457-57-3*0 2-Propynal cation [CHCCHO]+ (g) 1168.3 1167.2 ± 1.6 kJ/mol 54.0468 ± 100815-70-1*0 Formaldehyde CH2O (aq) -171.19 ± 0.59 kJ/mol 30.02598 ± 50-00-0*800 Dihydrogen anion [H2]- (g) 253.8 254.7 ± 1.2 kJ/mol 2.01643 ± 12184-89-3*0 Hydrogen peroxide cation [H2O2]+ (g, cis, para) 930.99 924.13 ± 0.98 kJ/mol 34.01413 ± 12325-10-9*22 Bicyclo[1.1.0]buta-1,3-diene-2,4-diyl C(CC)C (g, singlet) 1047.19 1054.78 ± 0.69 kJ/mol 48.0428 ± 12184-80-4*2 1-Methylethenyl anion [CH3CCH2]- (g) 199.4 188.3 ± 2.0 kJ/mol 41.0723 ± 65887-19-6*0 2-Hydroxyethylium [CH2CH2OH]+ (g, gauche) 1024.5 1011.9 ± 2.0 kJ/mol 45.0600 ± 38607-31-7*1 (Hydroxyimino)methyl HCNOH (g, trans-cis) 258.8 252.7 ± 2.0 kJ/mol 44.03272 ± 206442-92-4*2 Hydrogen peroxide cation [H2O2]+ (g, trans, ortho) 897.23 890.25 ± 0.51 kJ/mol 34.01413 ± 12325-10-9*11 Ammonium hydroxide NH4OH (aq, undissoc) -366.712 ± 0.061 kJ/mol 35.04584 ± 1336-21-6*1000 Cyanoacetylene cation [HCCCN]+ (g) 1495.43 1497.73 ± 0.62 kJ/mol 51.0462 ± 85398-68-1*0 Isocyanoacetylene cation [HCCNC]+ (g) 1567.7 1570.3 ± 1.3 kJ/mol 51.0462 ± 145076-03-5*0 1,3-Butadiyn-4-ylium-1-yl [CCCC]+ (g) 2110.0 2120.6 ± 2.8 kJ/mol 48.0423 ± 124708-46-9*0 Cyanoethynylium [CCCN]+ (g) 1867.6 1874.3 ± 3.2 kJ/mol 50.0383 ± 104233-69-4*0 Tetroxidane HOOOOH (g) -34.1 -44.6 ± 2.3 kJ/mol 66.0135 ± 29683-94-1*0 1,3-Butadiyne-1,4-diyl CCCC (g, triplet) 1052.40 1062.37 ± 0.66 kJ/mol 48.0428 ± 78015-07-3*1 Ethynylene C2 (g, singlet) 820.33 826.90 ± 0.26 kJ/mol 24.0214 ± 12070-15-4*2 Trioxygen difluoride FOOOF (g) 112.3 107.1 ± 1.1 kJ/mol 85.99501 ± 16829-28-0*0 Methyl formate HC(O)OCH3 (g) -346.88 -359.92 ± 0.84 kJ/mol 60.0520 ± 107-31-3*0 3-Imino-2-propen-1-ylidene CHCHCNH (g, singlet) 530.6 526.3 ± 1.2 kJ/mol 53.0627 ± 916765-05-4*1 1-Propenylium [CH3CHCH]+ (g, trans) 1233.9 1223.4 ± 2.0 kJ/mol 41.0713 ± 50457-58-4*1 Ethyl formate HC(O)OCH2CH3 (g) -375.3 -394.0 ± 1.2 kJ/mol 74.0785 ± 109-94-4*0 2-(Methylidyneamino)ethenyl CHCHNCH (g, singlet) 600.6 596.3 ± 1.2 kJ/mol 53.0627 ± 123416-74-0*1 1-Propenylide [CH3CHCH]- (g, trans) 210.6 199.4 ± 2.1 kJ/mol 41.0723 ± 65887-20-9*1 Hydroxylamine NH2OH (g) -33.12 -43.49 ± 0.45 kJ/mol 33.02996 ± 7803-49-8*0 Trihydrogen (H)(HH) (g, vdW) 215.9 218.3 ± 1.1 kJ/mol 3.02382 ± 12184-91-7*100 Oxygen atom dication [O]+2 (g) 4949.459 4953.000 ± 0.013 kJ/mol 15.99830 ± 14127-63-0*0 2-Butynylium [CH3CCCH2]+ (g, triplet) 1258.6 1251.3 ± 2.1 kJ/mol 53.0820 ± 64235-83-2*1 iso-Hexane CH3CH2CH2CH(CH3)2 (cr,l) -180.33 -203.99 ± 0.83 kJ/mol 86.1754 ± 107-83-5*500 Acetaldehyde CH3CHO (cr,l) -187.04 -191.76 ± 0.31 kJ/mol 44.0526 ± 75-07-0*500 Tetraoxygen O4 (g, D2h singlet) 69.8 65.1 ± 4.4 kJ/mol 63.9976 ± 12596-83-7*21 Hypochloroperoxoous acid, hydroxy ester HOOOCl (g, anti) 49.5 42.5 ± 2.5 kJ/mol 84.4588 ± 928652-99-7*2 Tetraoxygen O4 (g, D2d singlet) 399.0 393.4 ± 3.9 kJ/mol 63.9976 ± 12596-83-7*23 Tetraoxygen O4 (g, D3h singlet) 465.6 459.6 ± 4.2 kJ/mol 63.9976 ± 12596-83-7*24 2-Propen-1-ylium-1-ylidene [CH2CHC]+ (g) 1373.1 1369.5 ± 2.0 kJ/mol 39.0554 ± 88000-25-3*0 Acetoxy CH3C(O)O (g, 2A") -182.5 -191.3 ± 1.2 kJ/mol 59.0440 ± 13799-69-4*1 Nitrosyl nitrite anion [ONONO]- (g, t, c) -100.2 -103.4 ± 2.9 kJ/mol 76.01223 ± *122413-35-8*2 Deuteride D- (g) 146.991 148.904 ± 0.0021 kJ/mol 2.01465035791 ± 14452-69-8*0 Acetoxy CH3C(O)O (g) -182.5 -191.2 ± 1.2 kJ/mol 59.0440 ± 13799-69-4*0 Hydrogen peroxide H2O2 (g, para) -129.472 -135.458 ± 0.063 kJ/mol 34.01468 ± 7722-84-1*2 1,2-Butadien-1-ylium-1-yl [CH3CHCC]+ (g) 1466.8 1461.7 ± 3.2 kJ/mol 52.0740 ± 161054-21-3*0 Ethyl bromide CH3CH2Br (l) -55.52 -91.05 ± 0.26 kJ/mol 108.9651 ± 74-96-4*500 Bicyclo[1.1.0]butan-3-id-1-yl [CH2(CC)CH2]- (g) 524.6 515.9 ± 2.7 kJ/mol 52.0751 ± 131273-95-5*0 1,2-Cyclobutadiene C(CHCH2CH) (g) 586.0 579.4 ± 2.3 kJ/mol 52.0746 ± 50682-90-1*0 Dioxaziridine HN(OO) (g) 277.5 270.8 ± 1.9 kJ/mol 47.01348 ± 67394-35-8*0 Vinyl iodide CH2CHI (g) 139.6 130.8 ± 1.2 kJ/mol 153.9497 ± 593-66-8*0 Acetylene anion [HCCH]- (g) 356.8 356.7 ± 1.9 kJ/mol 26.0378 ± 50443-89-5*0 1,2-Propadien-1-ylium-1-yl-3-ylidene [CCC]+ (g) 1944.8 1952.7 ± 1.9 kJ/mol 36.0316 ± 118090-85-0*0 Propionic acid CH3CH2C(O)OH (g) -436.35 -454.24 ± 0.60 kJ/mol 74.0785 ± 79-09-4*0 Oxygen atom trication [O]+3 (g) 10249.929 10252.880 ± 0.018 kJ/mol 15.99775 ± 14127-64-1*0 Difluorovinylidene cation [CCF2]+ (g) 1181.6 1183.0 ± 2.3 kJ/mol 62.0177 ± 268566-71-8*0 Trihydrogen H(HH) (g, 2s 2A1' Ry) 745.878 743.094 ± 0.062 kJ/mol 3.02382 ± 12184-91-7*1 Deuterium molecule cation [D2]+ (g) 1492.286 1492.361 ± 0.000 kJ/mol 4.02765497609 ± 12184-84-8*0 Chlorine atom Cl (g, 2P3/2) 119.621 121.228 ± 0.0011 kJ/mol 35.45270 ± 22537-15-1*1 Trioxodinitrate anion [ONN(O)O]- (g, non-vdW) -87.3 -93.0 ± 2.2 kJ/mol 76.01223 ± 194856-62-7*1 Isocyanogen bromide anion [BrNC]- (g) 105.3 102.1 ± 4.6 kJ/mol 105.9220 ± *238754-53-5*0 Oxoniumyl ion [H2O]+ (g, ortho) 978.470 975.566 ± 0.028 kJ/mol 18.01473 ± 56583-62-1*1 Dioxosilane SiO2 (cr, tridymite) -900.3 -905.1 ± 1.5 kJ/mol 60.08430 ± 7631-86-9*515 Bromodioxy BrOO (g) 118.2 109.1 ± 4.3 kJ/mol 111.9028 ± 67177-47-3*0 Oxosilyliumyl [SiO]+ (g) 1022.2 1023.4 ± 3.0 kJ/mol 44.08435 ± 12359-18-1*0 Propylene cation [CH3CHCH2]+ (g) 975.17 961.64 ± 0.19 kJ/mol 42.0792 ± 34504-10-4*0 Oxonium [H3O]+ (g, para) 605.85 599.10 ± 0.17 kJ/mol 19.02267 ± 13968-08-6*2 Hydroxyoxoammoniumyl [HONO]+ (g, cis) 1014.8 1008.4 ± 2.0 kJ/mol 47.01293 ± 80371-48-8*2 Water trimer (H2O)3 (g) -762.72 -776.82 ± 0.20 kJ/mol 54.04584 ± 31014-12-7*0 Nitrous acid anion [HONO]- (g, trans) -105.4 -109.4 ± 1.8 kJ/mol 47.01403 ± 256393-36-9*1 Cyclopropane cation [CH2(CH2CH2)]+ (g) 1022.3 1006.8 ± 1.6 kJ/mol 42.0792 ± 34496-93-0*0 Silyloxoniumylidene [SiH3O]+ (g, triplet) 1096.2 1089.9 ± 2.9 kJ/mol 47.10817 ± 152720-17-7*1 Chlorodioxidenium [ClOO]+ (g) 1215.1 1212.9 ± 6.3 kJ/mol 67.4510 ± 64710-10-7*0 Hydroxysilylium [SiH2OH]+ (g) 600.2 591.5 ± 2.9 kJ/mol 47.10817 ± 66639-72-3*0 Fluorosyl fluoride FFO (g, singlet) 227. 225. ± 21. kJ/mol 53.99621 ± 86825-57-2*2 Difluorine cation [F2]+ (g) 1514.301 1514.475 ± 0.013 kJ/mol 37.9962578 ± 12184-86-0*0 Chloro(chlorooxy)methylene ClCOCl (g, trans triplet) 101.1 104.7 ± 4.8 kJ/mol 98.9155 ± 479587-31-0*12 1,5-Hexadiyne HCCCH2CH2CCH (cr,l) 385.8 ± 1.9 kJ/mol 78.1118 ± 628-16-0*500 Oxygen atom octacation [O]+8 (g) 197447.75 197449.60 ± 0.12 kJ/mol 15.99501 ± 21450-98-6*0 Iodine I (g, 2P1/2) 198.109 197.709 ± 0.0021 kJ/mol 126.904470 ± 14362-44-8*2 Dioxofluorine cation [OFO]+ (g) 1667.0 1664.8 ± 2.8 kJ/mol 50.99665 ± 223566-05-0*0 Acetylene HCCH (g, cis-triplet) 599.1 598.9 ± 1.5 kJ/mol 26.0373 ± 74-86-2*11 2-Oxo-1-ethylium-1-yl [HCC(O)H]+ (g, syn-quartet) 1292.0 1288.6 ± 2.4 kJ/mol 42.0361 ± 214464-96-7*12 Bicyclo[3.1.0]hexa-1,3-diene CH2((CCH)(CHCHCH)) (g) 406.3 390.7 ± 2.3 kJ/mol 78.1118 ± 28425-23-2*0 3-Ethenylidenecyclobutene CH2CC(CHCHCH2) (g) 406.4 392.3 ± 2.3 kJ/mol 78.1118 ± 813466-23-8*0 3,4-Hexadien-1-yne HCCCHCCHCH3 (g) 418.5 407.3 ± 2.6 kJ/mol 78.1118 ± 194024-51-6*0 3-Ethynylcyclobutene HCCCH(CHCHCH2) (g) 421.6 407.2 ± 2.4 kJ/mol 78.1118 ± 175090-90-1*0 Dinitrogen dimer (N2)2 (g) -0.88 0.84 ± 0.67 kJ/mol 56.02696 ± 154680-01-0*0 Polyoxymethylene CH2O (cr, polyoxymethylene) -170.88 ± 0.21 kJ/mol 30.02598 ± 9002-81-7*500 Oxoethylidene anion [HCC(O)H]- (g, syn-gauche) 92.3 89.0 ± 2.3 kJ/mol 42.0372 ± 106543-38-8*2 Hydroxylamine NH2OH (cr,l) -107.50 ± 0.62 kJ/mol 33.02996 ± 7803-49-8*500 Peroxyhypochlorous acid cation [HOOCl]+ (g, cis) 1050.8 1045.2 ± 2.5 kJ/mol 68.4589 ± *67952-07-2*2 Oxygen atom pentacation [O]+5 (g) 28708.788 28710.646 ± 0.053 kJ/mol 15.99666 ± 14127-66-3*0 Tribromine cation [Br3]+ (g) 1035.9 1013.2 ± 2.6 kJ/mol 239.7115 ± 33105-86-1*0 Dihydrogen cation [H2]+ (g, para) 1488.364 1488.480 ± 0.000 kJ/mol 2.01533 ± 12184-90-6*2 1,2-Diphenylethane C6H5CH2CH2C6H5 (g) 180.56 142.07 ± 0.76 kJ/mol 182.2610 ± 103-29-7*0 Isocyanogen cation [CNCN]+ (g) 1654.1 1656.5 ± 1.4 kJ/mol 52.0343 ± 133415-63-1*0 Chlorine trifluoride cation [FCl(F)F]+ (g) 1053.4 1049.1 ± 2.4 kJ/mol 92.44736 ± 478167-77-0*0 Carbon monoxide CO (g, singlet) -113.804 -110.524 ± 0.026 kJ/mol 28.01010 ± 630-08-0*2 Biphenyl C6H5C6H5 (l) 113.7 ± 1.1 kJ/mol 154.2078 ± 92-52-4*590 Buckminsterfullerene C60 (g) 2524.3 2519.6 ± 6.5 kJ/mol 720.6420 ± 99685-96-8*0 Buckminsterfullerene C60 (cr,l) 2329.2 2338.6 ± 6.5 kJ/mol 720.6420 ± 99685-96-8*500 Diisocyanogen cation [CNNC]+ (g, cis) 1838.6 1842.5 ± 3.1 kJ/mol 52.0343 ± *78800-21-2*2 Cyclobutatrienyl CH(CCC) (g) 961.9 965.4 ± 2.4 kJ/mol 49.0507 ± *99417-52-4*0 Dihydrogen H2 (g, para) -0.000 -0.058 ± 0.000 kJ/mol 2.01588 ± 1333-74-0*2 1-Propen-1-yl-3-ylidene CHCHCH (g) 656.6 653.2 ± 1.1 kJ/mol 39.0559 ± 84654-90-0*0 1,2-Butadiene CH3CHCCH2 (cr,l) 137.67 138.73 ± 0.66 kJ/mol 54.0904 ± 590-19-2*500 2-Cyclopropen-1-one cation [OC(CHCH)]+ (g) 1078.6 1075.5 ± 2.0 kJ/mol 54.0468 ± 100815-68-7*0 Phenol cation [C6H5OH]+ (g) 746.10 727.30 ± 0.64 kJ/mol 94.1107 ± 40932-22-7*0 Ethynol HCCOH (g, c-c triplet) 464.2 460.9 ± 3.2 kJ/mol 42.0367 ± 32038-79-2*15 Water H2O (cr, eq.press.) -286.300 -292.741 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*509 Oxonium [H3O]+ (g, ortho) 605.91 599.10 ± 0.17 kJ/mol 19.02267 ± 13968-08-6*1 Phenol anion [C6H5OH]- (g) 22.7 7.8 ± 4.9 kJ/mol 94.1118 ± 38752-11-3*0 Isourea NHC(OH)NH2 (g) -156.2 -172.8 ± 2.6 kJ/mol 60.05534 ± 4744-36-9*0 Hydroxyformyl HOCO (g) -181.14 -183.68 ± 0.44 kJ/mol 45.0174 ± 2564-86-5*0 Perchloric acid cation [HOCl(O)(O)O]+ (g) 1191.8 1182.2 ± 3.5 kJ/mol 100.4577 ± 91712-47-9*0 Carbamoyl anion [NH2CO]- (g) -81.9 -87.8 ± 3.1 kJ/mol 44.03327 ± 78944-69-1*0 Hydroxyformyl anion [HOCO]- (g) -319.53 -321.39 ± 0.57 kJ/mol 45.0180 ± 78944-70-4*0 Carbamoyl HNCOH (g) 74.1 68.3 ± 2.7 kJ/mol 44.03272 ± *2858-51-7*0 Carbamoyl HNCOH (g, trans-cis) 76.4 69.9 ± 3.2 kJ/mol 44.03272 ± *2858-51-7*2 Carbamoyl HNCOH (g, cis-trans) 96.3 90.1 ± 3.2 kJ/mol 44.03272 ± *2858-51-7*3 Carbamoyl HNCOH (g, cis-cis) 110.9 104.8 ± 3.2 kJ/mol 44.03272 ± *2858-51-7*4 2-Hydroxyethylium [CH2CH2OH]+ (g) 1019.1 1007.0 ± 1.5 kJ/mol 45.0600 ± 38607-31-7*0 Water dimer cation [(H2O)2]+ (g) 556.8 546.4 ± 3.0 kJ/mol 36.03001 ± 158061-35-9*0 Peroxychlorous acid HOOClO (g) 96.2 90.6 ± 2.1 kJ/mol 84.4588 ± 177778-92-6*0 Nitrosodioxaziridine ONN(OO) (g, cis) 413.9 408.1 ± 3.2 kJ/mol 76.01168 ± 132871-99-9*2 (Hydroxyimino)methyl HCNOH (g, cis-trans) 262.5 257.1 ± 2.5 kJ/mol 44.03272 ± 206442-92-4*3 (Hydroxyimino)methyl HCNOH (g, cis-cis) 281.4 275.9 ± 2.5 kJ/mol 44.03272 ± 206442-92-4*4 Hydrogen peroxide cation [H2O2]+ (g, trans, para) 897.26 890.25 ± 0.51 kJ/mol 34.01413 ± 12325-10-9*12 Dichloromethylene CCl2 (g) 229.51 230.69 ± 0.64 kJ/mol 82.9161 ± 1605-72-7*0 Hydroperoxyimidogen HOON (g, cis) 111.9 109.0 ± 2.8 kJ/mol 47.01348 ± 877456-58-1*2 Dichlorovinylidene CCCl2 (g) 421.7 424.9 ± 3.0 kJ/mol 94.9268 ± 20394-25-6*0 Tetraoxidanyl HOOOO (g, trans) 76.7 69.5 ± 6.1 kJ/mol 65.0055 ± 69696-93-1*1 Dioxymethylene HCOO (g) 341.8 339.8 ± 3.0 kJ/mol 45.0174 ± 438586-67-5*0 Formaldoxime CH2NOH (g) 30.5 19.9 ± 1.3 kJ/mol 45.04066 ± 75-17-2*0 2-Oxo-1-ethylium-1-yl [HCC(O)H]+ (g, quartet) 1289.4 1286.7 ± 2.5 kJ/mol 42.0361 ± 214464-96-7*1 1-Methoxyethenol CH2C(OH)OCH3 (g) -284.9 -303.3 ± 1.7 kJ/mol 74.0785 ± 4453-91-2*0 Chlorovinylidene CCHCl (g) 428.0 429.8 ± 3.0 kJ/mol 60.4820 ± 70277-82-6*0 Benzaldehyde cation [C6H5C(O)H]+ (g) 896.9 880.2 ± 1.9 kJ/mol 106.1214 ± 80226-59-1*0 Bromine atom Br (g, 2P1/2) 162.002 155.939 ± 0.056 kJ/mol 79.90400 ± 10097-32-2*2 Benzaldehyde anion [C6H5C(O)H]- (g) -60.2 -77.8 ± 1.2 kJ/mol 106.1225 ± 34473-57-9*0 Bicyclo[1.1.0]buta-1,3-diene-2,4-diyl C(CC)C (g, triplet) 1133.1 1140.9 ± 2.7 kJ/mol 48.0428 ± 12184-80-4*1 Hydrogen peroxide cation [H2O2]+ (g) 897.23 890.25 ± 0.51 kJ/mol 34.01413 ± 12325-10-9*0 Trihydrogen H(HH) (g, TS) 477.4 474.7 ± 2.3 kJ/mol 3.02382 ± 12184-91-7*201 Cyanogen chloride cation [ClCN]+ (g) 1326.05 1327.38 ± 0.97 kJ/mol 61.4696 ± 37612-72-9*0 2-Cyclopropyn-1-ylidene C(CC) (g) 898.9 905.8 ± 1.6 kJ/mol 36.0321 ± 102508-15-6*0 1-Propen-1-yl-3-ylidene CHCHCH (g, t-t quartet) 665.7 662.2 ± 2.0 kJ/mol 39.0559 ± 84654-90-0*13 Ethynol anion [HCCOH]- (g) 163.5 165.0 ± 3.3 kJ/mol 42.0372 ± 864971-50-6*0 Iodine monochloride ICl (cr,l) -36.511 -35.537 ± 0.063 kJ/mol 162.35717 ± 7790-99-0*500 Ethylene anion [CH2CH2]- (g) 232.0 225.5 ± 2.0 kJ/mol 28.0537 ± 34527-91-8*0 Nitrogen atom N (g, doublet) 700.555 702.458 ± 0.024 kJ/mol 14.006740 ± 17778-88-0*2 Oxirene O(CHCH) (g) 273.18 271.02 ± 0.92 kJ/mol 42.0367 ± 157-18-6*0 Peroxychlorous acid HOOClO (g, perp ?) 102.3 96.2 ± 3.7 kJ/mol 84.4588 ± 177778-92-6*3 3H-diazirin-3-ylidene-methylene CC(NN) (g) 837.3 839.9 ± 2.3 kJ/mol 52.0349 ± 204253-88-3*0 Ethane anion [CH3CH3]- (g) 41.9 26.6 ± 4.4 kJ/mol 30.0696 ± 54064-82-3*0 1-Cyanoethylidene CH3CCN (g) 437.5 433.7 ± 1.5 kJ/mol 53.0627 ± 498544-34-6*0 Difluorodioxidanium [FOOF]+ (g) 1283.7 1280.0 ± 2.3 kJ/mol 69.99506 ± *7783-44-0*0 Nitrosodioxaziridine ONN(OO) (g, gauche) 415.3 409.5 ± 2.8 kJ/mol 76.01168 ± 132871-99-9*3 Cyanic acid cation [HOCN]+ (g) 1135.7 1133.2 ± 2.9 kJ/mol 43.02423 ± 59780-31-3*0 Diazene HNNH (g, trans-cis equilib) 207.12 199.98 ± 0.41 kJ/mol 30.02936 ± 3618-05-1*1 Ethynol HCCOH (g, t-c triplet) 406.4 403.2 ± 3.2 kJ/mol 42.0367 ± 32038-79-2*12 Hydrazoic acid HNNN (cr,l) 261.51 ± 0.65 kJ/mol 43.02816 ± 7782-79-8*500 Difluorodioxidane anion [FOOF]- (g) -251. -251. ± 28. kJ/mol 69.99615 ± *7783-45-1*0 3-Imino-2-propen-1-ylidene CHCHCNH (g) 530.6 526.3 ± 1.2 kJ/mol 53.0627 ± 916765-05-4*0 Hydrogen cyanide anion [HCN]- (g) 129.513 129.035 ± 0.089 kJ/mol 27.02593 ± 12334-27-9*0 3-Imino-2-propen-1-ylidene CHCHCNH (g, triplet) 571.8 567.0 ± 1.6 kJ/mol 53.0627 ± 916765-05-4*2 1-Propen-1-yl-3-ylidene CHCHCH (g, doublet) 699.2 696.2 ± 3.4 kJ/mol 39.0559 ± 84654-90-0*2 Water H2O (g, ortho) -238.644 -241.832 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*1 Dichlorine cation [Cl2]+ (g) 1108.307 1108.286 ± 0.0062 kJ/mol 70.9049 ± 12595-90-3*0 Fluoromethane cation [CH3F]+ (g) 981.80 974.30 ± 0.62 kJ/mol 34.03237 ± 59122-96-2*0 2-(Methylidyneamino)ethenyl CHCHNCH (g) 600.6 596.3 ± 1.2 kJ/mol 53.0627 ± 123416-74-0*0 Fluorosyl fluoride anion [FFO]- (g) -147.1 -146.8 ± 3.0 kJ/mol 53.99675 ± *86825-57-2*0 2-(Methylidyneamino)ethenyl CHCHNCH (g, triplet) 634.5 630.4 ± 1.6 kJ/mol 53.0627 ± 123416-74-0*2 Cyanogen iodide cation [ICN]+ (g) 1271.4 1271.2 ± 1.6 kJ/mol 152.92136 ± 34749-78-5*0 Carbon C (diamond) 2.390 1.862 ± 0.044 kJ/mol 12.01070 ± 7440-44-0*501 Fluoromethane CH3F (l) -245.76 ± 0.45 kJ/mol 34.03292 ± 593-53-3*590 Diazene anion [HNNH]- (g, trans-cis-iso equilib) 265.5 258.7 ± 2.0 kJ/mol 30.02991 ± 100429-75-2*0 Diazoethenylidene cation [NNCC]+ (g) 1771.2 1773.2 ± 3.5 kJ/mol 52.0343 ± *130760-10-0*0 Dioxohydrazine ONNO (g, trans) 192.3 188.7 ± 1.8 kJ/mol 60.01228 ± 16824-89-8*1 Trihydrogen HHH (g, TS) 254.5 250.7 ± 1.2 kJ/mol 3.02382 ± 12184-91-7*200 Imidogen NH (g, triplet) 358.74 358.79 ± 0.16 kJ/mol 15.014680 ± 13774-92-0*1 Dicarbon monoxide CCO (g) 377.12 381.16 ± 0.83 kJ/mol 40.0208 ± 12071-23-7*0 1-Methylethylidene CH3CCH3 (g) 315.91 303.01 ± 0.99 kJ/mol 42.0797 ± 40852-89-9*0 Diazene anion [HNNH]- (g, trans-cis equilib) 265.5 258.7 ± 2.0 kJ/mol 30.02991 ± 100429-75-2*1 Carbonylmethyloxonium [CH3OCO]+ (g) 561.3 553.1 ± 3.2 kJ/mol 59.0435 ± 24434-00-2*0 Chloromethane CH3Cl (l) -106.10 -102.14 ± 0.25 kJ/mol 50.4872 ± 74-87-3*590 2-Cyanovinyl CHCHCN (g) 448.0 445.5 ± 1.2 kJ/mol 52.0547 ± 189141-20-6*0 2-Cyanovinylium [CHCHCN]+ (g, triplet) 1517.2 1515.1 ± 1.8 kJ/mol 52.0542 ± 85398-67-0*2 2-Cyanovinyl anion [CHCHCN]- (g) 263.8 261.4 ± 1.8 kJ/mol 52.0553 ± *85398-67-0*0 Methoxyoxomethyl CH3OCO (g) -152.6 -160.3 ± 1.5 kJ/mol 59.0440 ± 16481-04-2*0 mu-Oxodioxodinitrogen [ONONO]+ (g, t, t) 984.0 981.1 ± 3.2 kJ/mol 76.01113 ± 87950-63-8*1 2-Isocyanovinyl CHCHNC (g) 543.2 541.8 ± 1.7 kJ/mol 52.0547 ± 225798-14-1*0 Propylidene anion [CH3CH2CH]- (g) 300.8 286.6 ± 3.2 kJ/mol 42.0803 ± *121408-55-7*0 Fluorosyl fluoride cation [FFO]+ (g) 1413.1 1413.5 ± 3.5 kJ/mol 53.99566 ± *86825-50-5*0 Cyanomethyliumyl [HCCN]+ (g) 1506.7 1508.2 ± 2.3 kJ/mol 39.0355 ± 34447-38-6*0 Cyanomethylene anion [HCCN]- (g) 286.6 287.1 ± 1.5 kJ/mol 39.0366 ± 12373-24-9*0 Cyanomethylene HCCN (g) 479.9 483.3 ± 1.1 kJ/mol 39.0361 ± 2612-62-6*0 1,3-Butadiyne-1,4-diyl CCCC (g, singlet) 1084.14 1093.95 ± 0.84 kJ/mol 48.0428 ± 78015-07-3*2 Methoxyoxomethyl CH3OCO (g, syn-staggered) -151.9 -160.9 ± 2.2 kJ/mol 59.0440 ± 16481-04-2*2 Isocyanomethyliumyl [HCNC]+ (g) 1505.9 1507.8 ± 3.3 kJ/mol 39.0355 ± 99045-54-2*0 Isocyanomethylene anion [HCNC]- (g) 400.6 401.3 ± 2.1 kJ/mol 39.0366 ± 144141-01-5*0 Isocyanomethylene HCNC (g) 582.3 584.6 ± 1.7 kJ/mol 39.0361 ± 78694-65-2*0 Bromomethane cation [CH3Br]+ (g) 996.91 981.99 ± 0.27 kJ/mol 94.9380 ± 12538-70-4*0 3-Oxo-1,2-propadien-1-ylium-1-yl [CCCO]+ (g) 1379.9 1385.2 ± 3.9 kJ/mol 52.0310 ± 151455-13-9*0 3-Oxo-1,2-propadienylidene anion [CCCO]- (g) 223.9 229.7 ± 3.6 kJ/mol 52.0320 ± 109292-50-4*0 Pentachloroethylium [CCl3CCl2]+ (g) 836.2 834.5 ± 3.3 kJ/mol 201.2844 ± *7094-17-9*0 Nitrosyl nitrate ONONO2 (g) 48.3 40.4 ± 1.8 kJ/mol 92.0111 ± 15969-55-8*0 Methoxyoxomethyl CH3OCO (g, anti-eclipsed) -151.7 -159.8 ± 2.2 kJ/mol 59.0440 ± 16481-04-2*3 Cyanic acid anion [HOCN]- (g) -9.2 -9.6 ± 3.1 kJ/mol 43.02533 ± 58199-47-6*0 Hydrogen isocyanide anion [HNC]- (g) 191.60 191.79 ± 0.33 kJ/mol 27.02593 ± 96913-22-3*0 Diazanide [NH2NH]- (g) 249.3 239.3 ± 3.1 kJ/mol 31.03785 ± 25415-88-7*0 Propylidyne CH3CH2C (g) 492.5 482.8 ± 1.2 kJ/mol 41.0718 ± 85056-54-8*0 Pentachloroethanide [CCl3CCl2]- (g) -283.7 -282.2 ± 3.9 kJ/mol 201.2854 ± 263019-83-6*0 O--HH Complex (H2)(O) (g, vdW C2v ?) 247.2 248.2 ± 2.0 kJ/mol 18.01528 ± *7732-18-5*102 Fluorosyl fluoride FFO (g) 192.8 193.3 ± 2.3 kJ/mol 53.99621 ± 86825-57-2*0 Oxoethylidene anion [HCC(O)H]- (g) 86.9 84.2 ± 2.1 kJ/mol 42.0372 ± 106543-38-8*0 Nitrosodioxaziridine ONN(OO) (g) 413.7 408.2 ± 1.8 kJ/mol 76.01168 ± 132871-99-9*0 1,3-Cyclobutadiene cation [CH(CHCHCH)]+ (g) 1215.5 1207.2 ± 3.1 kJ/mol 52.0740 ± 34531-09-4*0 Acetyloxoniumylidene [CH3C(O)O]+ (g, triplet) 891.9 883.5 ± 3.0 kJ/mol 59.0435 ± 67057-47-0*1 Formic acid cation [HC(O)OH]+ (g) 721.89 715.36 ± 0.26 kJ/mol 46.0248 ± 50614-05-6*0 2-Methyl-2-cyclopropen-1-ylidene CH3C(CHC) (g) 447.9 441.7 ± 1.2 kJ/mol 52.0746 ± 185841-59-2*0 2-Methyl-2-cyclopropen-1-ylidene CH3C(CHC) (g, triplet) 670.5 664.5 ± 2.8 kJ/mol 52.0746 ± 185841-59-2*1 Ethynol HCCOH (g) 95.31 92.82 ± 0.80 kJ/mol 42.0367 ± 32038-79-2*0 Iodomethane cation [CH3I]+ (g) 944.80 935.40 ± 0.18 kJ/mol 141.93844 ± 12538-72-6*0 Hydroperoxonium [H2OOH]+ (g, cis) 762.5 752.7 ± 2.0 kJ/mol 35.02207 ± 70850-26-9*2 Isocyanomethane CH3NC (cr,l) 146.5 ± 2.2 kJ/mol 41.0520 ± 593-75-9*500 Peroxyformic acid HC(O)OOH (g) -279.24 -288.38 ± 0.88 kJ/mol 62.0248 ± 107-32-4*0 Diazene cation [HNNH]+ (g, trans-cis equilib) 1132.49 1125.55 ± 0.64 kJ/mol 30.02881 ± 76986-17-9*1 Vinylvinylidene CH2CHCHC (g) 494.1 488.7 ± 1.8 kJ/mol 52.0746 ± 148759-16-4*0 Vinylvinylidene CH2CHCHC (g, triplet) 690.4 684.6 ± 2.7 kJ/mol 52.0746 ± 148759-16-4*1 Ethylidene CH3CH (g) 361.20 354.22 ± 0.51 kJ/mol 28.0532 ± 4218-50-2*0 Vinylvinylidene CH2CHCHC (g, triplet, cis) 695.4 688.9 ± 3.3 kJ/mol 52.0746 ± 148759-16-4*12 Vinylvinylidene cation [CH2CHCHC]+ (g) 1429.7 1424.4 ± 2.1 kJ/mol 52.0740 ± 161054-20-2*0 Vinylvinylidene cation [CH2CHCHC]+ (g, cis) 1437.7 1432.0 ± 2.7 kJ/mol 52.0740 ± 161054-20-2*2 Vinylvinylidene anion [CH2CHCHC]- (g) 405.7 398.9 ± 2.1 kJ/mol 52.0751 ± 167386-49-4*0 Vinylvinylidene anion [CH2CHCHC]- (g, cis) 423.2 416.6 ± 2.7 kJ/mol 52.0751 ± 167386-49-4*2 Ethenol CH2CHOH (g) -113.11 -123.49 ± 0.52 kJ/mol 44.0526 ± 557-75-5*0 Acetone enol CH3C(OH)CH2 (g) -152.0 -168.8 ± 1.1 kJ/mol 58.0791 ± 29456-04-0*0 1,2-Butadien-1-ylidene CH3CHCC (g) 511.7 506.2 ± 2.1 kJ/mol 52.0746 ± 119908-55-3*0 1,2-Butadien-1-ylidene CH3CHCC (g, triplet) 651.7 646.2 ± 3.7 kJ/mol 52.0746 ± 119908-55-3*1 Dioxodiazoxane ONONO (g) 93.0 88.1 ± 1.3 kJ/mol 76.01168 ± 122413-35-8*0 Formic acid anion [HC(O)OH]- (g) -256.2 -262.3 ± 1.8 kJ/mol 46.0259 ± 19066-82-1*0 Nitrogen atom anion N- (g) 487.6 489.9 ± 2.5 kJ/mol 14.007289 ± 17778-87-9*0 Hydrogen peroxide cation [H2O2]+ (g, cis, ortho) 931.01 924.13 ± 0.98 kJ/mol 34.01413 ± 12325-10-9*21 2-Hydroxyethyl CH2CH2OH (g) -13.37 -26.63 ± 0.56 kJ/mol 45.0605 ± 4422-54-2*0 Vinyl alcohol cation [CH2CHOH]+ (g) 778.7 767.7 ± 1.3 kJ/mol 44.0520 ± 57239-63-1*0 Peroxyformyl HC(O)OO (g) -100.37 -105.47 ± 0.92 kJ/mol 61.0168 ± 56240-83-6*0 Hydrazine anion [NH2NH2]- (g) 232.3 217.9 ± 4.1 kJ/mol 32.04579 ± 65391-88-0*0 (Formyloxy)methyl HC(O)OCH2 (g) -151.8 -159.9 ± 1.3 kJ/mol 59.0440 ± 3122-12-1*0 1,2,3-Butatrien-1-ylium [CH2CCCH]+ (g) 1271.7 1269.9 ± 1.1 kJ/mol 51.0661 ± 81932-79-8*0 Nitrogen sesquioxide cation [ONN(O)O]+ (g, vdW) 1027.7 1023.7 ± 4.8 kJ/mol 76.01113 ± *10544-73-7*0 Carboxymethyl CH2C(O)OH (g) -227.2 -237.0 ± 1.2 kJ/mol 59.0440 ± 2887-46-9*0 Oxiranylidene CH2(CO) (g) 213.5 209.2 ± 1.4 kJ/mol 42.0367 ± 50983-85-2*0 Tetraoxidanyl HOOOO (g) 2.0 0.2 ± 3.0 kJ/mol 65.0055 ± 69696-93-1*0 Hydrobromate ion [HBr]- (g) -5.6 -12.1 ± 4.3 kJ/mol 80.9125 ± 85532-97-4*0 2-Methoxy-2-oxoethyl CH2C(O)OCH3 (g) -203.4 -218.6 ± 1.6 kJ/mol 73.0706 ± 54668-31-4*0 1-Buten-3-yn-1-yl HCCCHCH (g) 547.17 544.66 ± 0.88 kJ/mol 51.0666 ± 2810-61-9*0 1-Buten-3-ynylium [HCCCHCH]+ (g) 1482.3 1480.1 ± 1.5 kJ/mol 51.0661 ± 84654-85-3*0 But-1-en-3-yn-1-ide [HCCCHCH]- (g) 419.7 419.1 ± 1.3 kJ/mol 51.0672 ± 119611-65-3*0 Methoxyethene CH2CHOCH3 (g) -89.3 -106.6 ± 1.1 kJ/mol 58.0791 ± 107-25-5*0 Imidogen NH (g, singlet) 509.34 509.39 ± 0.17 kJ/mol 15.014680 ± 13774-92-0*2 Hypochloroperoxoous acid, hydroxy ester HOOOCl (g) 49.2 42.4 ± 1.9 kJ/mol 84.4588 ± 928652-99-7*0 3-Buten-1-ynylium [CH2CHCC]+ (g) 1572.5 1570.1 ± 1.7 kJ/mol 51.0661 ± 103928-84-3*0 Chlorine pentafluoride anion [FCl(F)(F)(F)F]- (g) -619.3 -622.7 ± 4.4 kJ/mol 130.44526 ± 184851-42-1*0 Chlorine atom Cl (g, 2P1/2) 130.176 131.783 ± 0.0011 kJ/mol 35.45270 ± 22537-15-1*2 (Acetyloxy)methyl CH3C(O)OCH2 (g) -200.6 -214.3 ± 1.6 kJ/mol 73.0706 ± 2887-49-2*0 Fluoroform CHF3 (l) -704.46 ± 0.43 kJ/mol 70.01385 ± 75-46-7*590 3-Methylene-1-cyclopropen-1-ylium [CH2C(CHC)]+ (g) 1398.6 1395.3 ± 1.8 kJ/mol 51.0661 ± 117469-28-0*0 Hydroxyamidogen HNOH (g) 101.37 94.53 ± 0.81 kJ/mol 32.02202 ± 13940-32-4*0 Isocyanogen chloride anion [ClNC]- (g) 102.8 107.2 ± 3.9 kJ/mol 61.4707 ± 923019-39-0*0 2-Butynylium [CH3CCCH2]+ (g) 1085.6 1077.4 ± 1.6 kJ/mol 53.0820 ± 64235-83-2*0 2-Cyclopropen-1-ylidenemethylium [CHC(CHCH)]+ (g) 1346.3 1343.7 ± 1.8 kJ/mol 51.0661 ± 112936-36-4*0 1,2-Difluoroethene CHFCHF (g) -302.90 -309.00 ± 0.90 kJ/mol 64.0341 ± 1691-13-0*0 1,2-Propadien-1-yl-3-ylidene anion [CCC]- (g, lin) 622.0 629.4 ± 1.3 kJ/mol 36.0326 ± 109292-47-9*1 Chloromethylene CHCl (g) 320.27 320.55 ± 0.89 kJ/mol 48.4713 ± 2108-20-5*0 Methoxyethene CH2CHOCH3 (g, trans) -81.9 -99.0 ± 2.4 kJ/mol 58.0791 ± 107-25-5*1 1,3-Cyclobutadien-1-ylium [C(CHCHCH)]+ (g) 1388.2 1383.2 ± 1.9 kJ/mol 51.0661 ± 81913-77-1*0 Hydroxyaminylium [HNOH]+ (g) 1019.5 1012.4 ± 2.5 kJ/mol 32.02147 ± 66097-71-0*0 2-Hydroxyethenyl CHCHOH (g) 138.01 132.18 ± 0.92 kJ/mol 43.0446 ± 108665-16-3*0 C=O-C cation [COC]+ (g) 1585.8 1592.9 ± 2.7 kJ/mol 40.0203 ± 83483-98-1*0 Cyclobutenide [CH2(CHCHCH)]- (g) 318.8 306.3 ± 3.7 kJ/mol 53.0830 ± 60211-42-9*0 Isocyanogen chloride cation [ClNC]+ (g) 1480.8 1482.1 ± 3.9 kJ/mol 61.4696 ± 956475-91-5*0 Methyl nitrite CH3ONO (g, trans) -52.40 -64.36 ± 0.89 kJ/mol 61.0401 ± 624-91-9*1 Difluorovinylidene anion [CCF2]- (g) -88.2 -87.1 ± 2.3 kJ/mol 62.0188 ± 107693-92-5*0 Methane anion [CH4]- (g) 27.3 19.4 ± 5.1 kJ/mol 16.04301 ± 27680-53-1*0 Phenylperoxy cation [C6H5OO]+ (g) 1018.2 1001.2 ± 3.1 kJ/mol 109.1022 ± 956036-17-2*0 Phenylperoxy anion [C6H5OO]- (g) -39.6 -56.6 ± 3.3 kJ/mol 109.1032 ± *956036-17-2*0 Ethoxyethene CH2CHOCH2CH3 (g) -117.7 -136.2 ± 1.5 kJ/mol 72.1057 ± 109-92-2*0 Oxonium [H3O]+ (aq) -285.826 ± 0.026 kJ/mol 19.02267 ± 13968-08-6*800 Dibromovinylidene CCBr2 (g) 511.0 499.9 ± 4.1 kJ/mol 183.8294 ± 261771-36-2*0 Fluorovinylidene CCHF (g) 298.9 300.1 ± 3.1 kJ/mol 44.0277 ± 70302-00-0*0 Methyl nitrite CH3ONO (cr,l) -88.75 ± 0.56 kJ/mol 61.0401 ± 624-91-9*500 Fulvene cation [CH2C(CHCHCHCH)]+ (g) 1043.4 1027.6 ± 2.7 kJ/mol 78.1113 ± 34504-75-1*0 Fulvene anion [CH2C(CHCHCHCH)]- (g) 191.2 176.1 ± 4.0 kJ/mol 78.1124 ± 34504-63-7*0 Isocyanogen bromide cation [BrNC]+ (g) 1470.2 1464.5 ± 5.9 kJ/mol 105.9209 ± 238754-53-5*0 Methylaminyliumyl [CH3N]+ (g ?) 1388.3 1381.9 ± 2.3 kJ/mol 29.04071 ± 56924-51-7*0 Methanediol CH2(OH)2 (g) -379.36 -392.75 ± 0.92 kJ/mol 48.0413 ± 463-57-0*0 Ethyl nitrite CH3CH2ONO (g, gauche-trans) -80.3 -98.5 ± 1.9 kJ/mol 75.0666 ± 109-95-5*2 Silicon cation Si+ (g) 1237.04 1241.17 ± 0.64 kJ/mol 28.08495 ± 14067-07-3*0 Silicon anion Si- (g) 316.46 319.44 ± 0.64 kJ/mol 28.08605 ± 14337-02-1*0 Ethyl nitrite CH3CH2ONO (g, gauche-cis) -78.3 -97.2 ± 1.9 kJ/mol 75.0666 ± 109-95-5*3 Bromovinylidene CCHBr (g) 466.6 460.6 ± 3.8 kJ/mol 104.9333 ± 161957-27-3*0 Disilicon anion [Si2]- (g) 375.5 379.4 ± 1.9 kJ/mol 56.17155 ± 64236-73-3*0 Nitrato [ON(O)O]+ (g) 1290.62 1285.91 ± 0.86 kJ/mol 62.00439 ± 133057-96-2*0 Diazene cation [HNNH]+ (g, trans-iso-cis equilib) 1132.49 1125.83 ± 0.64 kJ/mol 30.02881 ± 76986-17-9*0 Difluorophosgene anion [CF2O]- (g) -602.3 -604.2 ± 3.3 kJ/mol 66.00745 ± 474815-26-4*0 Ethoxyethene CH2CHOCH2CH3 (g, cis-perp) -111.3 -135.1 ± 2.3 kJ/mol 72.1057 ± 109-92-2*21 Trioxidaniumyl [HOOOH]+ (g) 925.5 916.6 ± 1.5 kJ/mol 50.01353 ± 161321-37-5*0 Isocyanic fluoride anion [FNC]- (g) 74.3 75.9 ± 5.7 kJ/mol 45.01639 ± 357426-78-9*0 Propyl nitrite CH3CH2CH2ONO (g) -96.1 -119.9 ± 1.8 kJ/mol 89.0932 ± 543-67-9*0 Diiodine cation [I2]+ (g) 963.523 960.408 ± 0.018 kJ/mol 253.808391 ± 28712-14-3*0 Propyl nitrite CH3CH2CH2ONO (g, trans-trans-cis) -95.8 -120.4 ± 2.5 kJ/mol 89.0932 ± 543-67-9*2 Propyl nitrite CH3CH2CH2ONO (g, gauche-gauche-trans) -96.0 -120.5 ± 2.5 kJ/mol 89.0932 ± 543-67-9*3 Propyl nitrite CH3CH2CH2ONO (g, gauche-gauche-trans II) -95.2 -119.8 ± 2.5 kJ/mol 89.0932 ± 543-67-9*4 Cyanomethylium [CH2CN]+ (g) 1256.77 1254.13 ± 0.74 kJ/mol 40.0435 ± 34430-18-7*0 Fluoro(fluorooxy)methylene FCOF (g) -134.8 -136.0 ± 1.3 kJ/mol 66.00691 ± 479587-30-9*0 Silicon carbide anion [SiC]- (g) 510.0 514.6 ± 4.4 kJ/mol 40.09675 ± 86017-99-4*0 Diazenide [NNH]- (g) 175.6 176.7 ± 1.6 kJ/mol 29.02197 ± 71004-29-0*0 Propyl nitrite CH3CH2CH2ONO (g, trans-gauche-trans) -95.1 -119.4 ± 2.5 kJ/mol 89.0932 ± 543-67-9*5 Ethoxyethene CH2CHOCH2CH3 (g, trans-trans) -110.7 -132.9 ± 2.6 kJ/mol 72.1057 ± 109-92-2*1 Silicon carbide SiC (cr,l) -70.5 -71.5 ± 1.5 kJ/mol 40.09620 ± 409-21-2*500 Propyl nitrite CH3CH2CH2ONO (g, trans-gauche-cis) -93.2 -118.2 ± 2.5 kJ/mol 89.0932 ± 543-67-9*6 Chlorohydroxymethyl CH(Cl)OH (g) -54.7 -60.5 ± 1.1 kJ/mol 65.4787 ± 147139-06-8*0 Propyl nitrite CH3CH2CH2ONO (g, gauche-gauche-cis) -92.9 -118.1 ± 2.5 kJ/mol 89.0932 ± 543-67-9*7 Oxoiodonium [IO]+ (g) 1066.1 1064.1 ± 1.9 kJ/mol 142.90332 ± 81182-81-2*0 Ethoxyethene CH2CHOCH2CH3 (g, trans-perp) -108.7 -131.9 ± 2.3 kJ/mol 72.1057 ± 109-92-2*11 Propyl nitrite CH3CH2CH2ONO (g, gauche-gauche-cis II) -91.5 -116.4 ± 2.8 kJ/mol 89.0932 ± 543-67-9*8 Peroxyhypochlorous acid cation [HOOCl]+ (g) 1030.3 1024.5 ± 2.5 kJ/mol 68.4589 ± *67952-07-2*0 Oxiranylium [CH2(CHO)]+ (g) 901.2 893.2 ± 1.5 kJ/mol 43.0441 ± 57789-76-1*0 Dioxoethylium [OCHCO]+ (g) 688.2 689.5 ± 3.1 kJ/mol 57.0276 ± *1994332-01-2*0 Silylene SiH2 (g) 272.5 270.8 ± 1.2 kJ/mol 30.10138 ± 13825-90-6*0 Ethanedial anion [OCHCO]- (g) -189.1 -190.6 ± 3.3 kJ/mol 57.0287 ± 1994332-01-2*0 Isoformyl anion [COH]- (g) 55.7 58.4 ± 1.8 kJ/mol 29.01859 ± 81747-63-9*0 1,2-Dibromoethene CHBrCHBr (g) 121.2 101.1 ± 2.0 kJ/mol 185.8453 ± 540-49-8*0 Oxiranyl anion [CH2(CHO)]- (g) 121.2 113.3 ± 1.5 kJ/mol 43.0452 ± 72722-59-9*0 Disilane cation [SiH3SiH3]+ (g) 1030.5 1017.7 ± 2.3 kJ/mol 62.21809 ± 96301-43-8*0 Hydrogen peroxide H2O2 (aq) -190.75 ± 0.30 kJ/mol 34.01468 ± 7722-84-1*800 Propynylidene HCCCH (g, singlet) 597.6 599.4 ± 1.3 kJ/mol 38.0480 ± 67152-18-5*2 Hydroxyoxoethenylium [HOCCO]+ (g) 866.2 865.0 ± 3.5 kJ/mol 57.0276 ± *1994332-00-1*0 Ethynediolide [HOCCO]- (g) -133.1 -134.2 ± 3.5 kJ/mol 57.0287 ± 1994332-00-1*0 Phosgene cation [CCl2O]+ (g) 897.1 896.0 ± 1.7 kJ/mol 98.9150 ± 73249-38-4*0 Chlorous acid cation [HOClO]+ (g) 996.6 991.6 ± 3.1 kJ/mol 68.4589 ± *99559-66-7*0 Phosgene CCl2O (cr, l, I+liquid) -242.74 ± 0.27 kJ/mol 98.9155 ± 75-44-5*501 Cyclopropylidene C(CH2CH2) (g) 474.87 466.84 ± 0.85 kJ/mol 40.0639 ± 2143-70-6*0 Phosgene CCl2O (cr, l, II+liquid) -255.44 -242.74 ± 0.27 kJ/mol 98.9155 ± 75-44-5*502 Chlorine trifluoride anion [FCl(F)F]- (g) -480.4 -481.9 ± 3.6 kJ/mol 92.44846 ± 184289-50-7*0 Trihydrogen H(HH) (g, Ry) 745.878 743.199 ± 0.062 kJ/mol 3.02382 ± 12184-91-7*0 Chlorine chlorite ClOClO (g) 168.6 166.4 ± 1.3 kJ/mol 102.9042 ± 105206-44-8*0 Chloro(chlorooxy)methylene ClCOCl (g) 73.5 73.9 ± 3.6 kJ/mol 98.9155 ± 479587-31-0*0 Ethynol HCCOH (g, t-t triplet) 409.4 406.3 ± 3.2 kJ/mol 42.0367 ± 32038-79-2*13 Oxoethylidene HCC(O)H (g) 261.1 258.3 ± 1.1 kJ/mol 42.0367 ± 39920-84-8*0 Hydroxysilyl anion [SiH2OH]- (g, anti) -218.9 -226.8 ± 2.8 kJ/mol 47.10927 ± 78450-47-2*2 Chloro(chlorooxy)methylene ClCOCl (g, trans) 98. 105. ± 15. kJ/mol 98.9155 ± 479587-31-0*1 (Methylamino)methyl anion [CH3NHCH2]- (g) 201.2 184.3 ± 3.2 kJ/mol 44.0763 ± *31277-25-5*0 Chloroethane CH3CH2Cl (cr,l) -134.30 -135.92 ± 0.27 kJ/mol 64.5138 ± 75-00-3*500 2,2,4-Trimethylpentane (CH3)2CHCH2C(CH3)3 (g) -171.3 -223.7 ± 1.5 kJ/mol 114.2285 ± 540-84-1*0 Benzvalene cation [((CHCH)(CH(CHCH)CH) (g) 1200.1 1184.7 ± 2.7 kJ/mol 78.1113 ± 81050-63-7*0 Cyanic fluoride cation [FCN]+ (g) 1294.4 1294.9 ± 1.1 kJ/mol 45.01529 ± 62532-69-8*0 Cyanic fluoride anion [FCN]- (g) -20.4 -18.4 ± 2.9 kJ/mol 45.01639 ± 34507-50-1*0 Cyclopropane CH2(CH2CH2) (l) 41.41 35.72 ± 0.53 kJ/mol 42.0797 ± 75-19-4*500 Oxirene O(CHCH) (g, triplet) 387.8 384.5 ± 1.4 kJ/mol 42.0367 ± 157-18-6*1 Diazoethenylidene anion [NNCC]- (g) 529.7 532.3 ± 3.5 kJ/mol 52.0354 ± *130760-11-1*0 Water anion [H2O]- (g) -50.1 -53.0 ± 4.3 kJ/mol 18.01583 ± 12259-30-2*0 Peroxyformate [HC(O)OO]- (g) -340.91 -346.36 ± 0.95 kJ/mol 61.0174 ± 104960-05-6*0 Peroxyhypochlorous acid anion [HOOCl]- (g, quartet) -32.2 -34.0 ± 2.6 kJ/mol 68.4600 ± *67952-08-3*0 Oxoniumyl ion [H2O]+ (g, para) 978.719 975.569 ± 0.028 kJ/mol 18.01473 ± 56583-62-1*2 Chlorohydroxymethylene ClCOH (g, cis) 14.3 11.4 ± 3.2 kJ/mol 64.4707 ± 147723-60-2*2 Chlorohydroxymethylene ClCOH (g) 13.9 11.2 ± 2.6 kJ/mol 64.4707 ± 147723-60-2*0 Butadienylacetylene CH2CHCHCHCCH (g) 358.7 346.2 ± 1.9 kJ/mol 78.1118 ± 10420-90-3*0 Cyclopentadienide [CH(CHCHCHCH)]- (g) 100.2 87.2 ± 1.1 kJ/mol 65.0937 ± 12127-83-2*0 (3Z)-1,3-Hexadien-5-yne CH2CHCHCHCCH (g, cis) 358.7 346.1 ± 2.5 kJ/mol 78.1118 ± 5222-76-4*0 Divinylacetylene CH2CHCCCHCH2 (g) 361.7 350.6 ± 2.0 kJ/mol 78.1118 ± 821-08-9*0 Ethynol cation [HCCOH]+ (g) 1063.8 1061.2 ± 2.9 kJ/mol 42.0361 ± 84005-02-7*0 Divinylacetylene CH2CHCCCHCH2 (g, trans) 362.3 351.2 ± 2.6 kJ/mol 78.1118 ± 821-08-9*1 Biallenyl CH2CCHCHCCH2 (g) 410.5 399.4 ± 1.5 kJ/mol 78.1118 ± 29776-96-3*0 Nitrous acid anion [HONO]- (g) -105.84 -109.31 ± 0.78 kJ/mol 47.01403 ± 256393-36-9*0 Biallenyl CH2CCHCHCCH2 (g, cis) 419.3 408.0 ± 2.3 kJ/mol 78.1118 ± 29776-96-3*2 Propargylallene CH2CCHCH2CCH (g) 431.2 419.8 ± 1.4 kJ/mol 78.1118 ± 33142-15-3*0 Trihydrogen cation [H3]+ (g, ortho) 1110.577 1106.695 ± 0.013 kJ/mol 3.02327 ± 28132-48-1*1 Propargylallene CH2CCHCH2CCH (g, cis) 432.1 420.5 ± 2.2 kJ/mol 78.1118 ± 33142-15-3*2 Hydrazoic acid anion [HNNN]- (g) 309.0 303.2 ± 2.3 kJ/mol 43.02871 ± 203264-98-6*0 (Chlorooxy)methylene HCOCl (g, trans) 155.4 155.2 ± 3.7 kJ/mol 64.4707 ± 331419-80-8*1 3-Methylene-1-penten-4-yne CH2CHC(CH2)CCH (g) 362.5 350.1 ± 2.2 kJ/mol 78.1118 ± 6929-96-0*0 3-Methylene-1-penten-4-yne CH2CHC(CH2)CCH (g, gauche) 374.6 362.3 ± 2.8 kJ/mol 78.1118 ± 6929-96-0*3 1,2,3,5-Hexatetraene CH2CCCHCHCH2 (g) 391.6 380.2 ± 1.6 kJ/mol 78.1118 ± 60239-33-0*0 Isocyanic fluoride cation [FNC]+ (g) 1618.4 1619.0 ± 4.0 kJ/mol 45.01529 ± *16992-26-0*0 1,2,3,5-Hexatetraene CH2CCCHCHCH2 (g, cis) 400.4 388.8 ± 2.3 kJ/mol 78.1118 ± 60239-33-0*2 (Chlorooxy)methylene HCOCl (g) 119.1 117.8 ± 2.7 kJ/mol 64.4707 ± 331419-80-8*0 Isofulminic acid cation [HONC]+ (g) 1376.9 1374.8 ± 2.9 kJ/mol 43.02423 ± 117800-60-9*0 (Dimethylamino)methyl anion [(CH3)2NCH2]- (g) 190.2 166.0 ± 3.3 kJ/mol 58.1029 ± 125231-49-4*0 Diazene HNNH (g, trans-cis-iso equilib) 207.12 199.98 ± 0.41 kJ/mol 30.02936 ± 3618-05-1*0 Trioxygen difluoride FOOOF (g, cis) 113.4 107.6 ± 2.1 kJ/mol 85.99501 ± 16829-28-0*2 Formylimidogen HC(O)N (g, triplet) 245.2 242.1 ± 4.6 kJ/mol 43.02478 ± 42288-92-6*1 Nitrosyl nitrite anion [ONONO]- (g) -103.0 -106.1 ± 2.8 kJ/mol 76.01223 ± *122413-35-8*0 trans-Cycloheptene CH2(CHCHCH2CH2CH2CH2 (g) 141.6 103.6 ± 1.9 kJ/mol 96.1702 ± 45509-99-7*0 1-Propen-1-yl-3-ylidene CHCHCH (g, c-t quartet) 656.6 653.1 ± 2.0 kJ/mol 39.0559 ± 84654-90-0*12 2-Propenylidene CHCHCH2 (g, triplet) 417.4 410.4 ± 1.5 kJ/mol 40.0639 ± 19527-08-3*1 Dioxyamidogen HNOO (g, trans) 251.1 244.6 ± 2.5 kJ/mol 47.01348 ± 71843-95-3*1 Hydrodioxonitrogen cation [HN(O)O]+ (g) 1130.2 1123.6 ± 3.0 kJ/mol 47.01293 ± 252986-76-8*0 (Z)-1-Methylallyl CH3CHCHCH2 (g, cis) 157.2 139.8 ± 2.1 kJ/mol 55.0984 ± 98705-00-1*0 1-Methylallyl CH3CHCHCH2 (g) 154.61 138.14 ± 0.79 kJ/mol 55.0984 ± 15819-46-2*0 1,1-Dibromoethane CH3CHBr2 (cr,l) -67.1 ± 1.3 kJ/mol 187.8612 ± 557-91-5*500 Bromoform CHBr3 (l) 3.5 ± 1.3 kJ/mol 252.7306 ± 75-25-2*590 Chloroformyl anion [ClCO]- (g) -356.5 -352.9 ± 3.3 kJ/mol 63.4633 ± 41976-93-6*0 2-Propenylidene CHCHCH2 (g) 417.4 410.4 ± 1.5 kJ/mol 40.0639 ± 19527-08-3*0 Aminomethylidyne anion [CNH2]- (g, triplet) 481.6 478.0 ± 3.2 kJ/mol 28.03387 ± 96808-04-7*1 Ethynol HCCOH (g, c-t triplet) 455.5 452.2 ± 3.2 kJ/mol 42.0367 ± 32038-79-2*14 1,2-Dichloroethene CHClCHCl (g) 3.47 -1.85 ± 0.54 kJ/mol 96.9427 ± 540-59-0*0 N-Methylethanamine CH3CH2NHCH3 (cr,l) -75.2 ± 2.3 kJ/mol 59.1103 ± 624-78-2*500 Hydrogen peroxide H2O2 (g, ortho) -129.451 -135.458 ± 0.063 kJ/mol 34.01468 ± 7722-84-1*1 Iminomethyl HCNH (g) 275.79 272.19 ± 0.61 kJ/mol 28.03332 ± 15691-95-9*0 Hydroxymethylene anion [HCOH]- (g) 147.1 144.2 ± 1.6 kJ/mol 30.02653 ± 207914-87-2*0 2-Methyl-2-propanamine (CH3)3CNH2 (l) -150.74 ± 0.54 kJ/mol 73.1369 ± 75-64-9*590 3-Buten-2-one CH3C(O)CHCH2 (g) -95.49 -111.02 ± 0.87 kJ/mol 70.0898 ± 78-94-4*0 Cyanogen NCCN (cr,l) 273.93 289.18 ± 0.42 kJ/mol 52.0349 ± 460-19-5*500 2-Propenylidene CHCHCH2 (g, triplet syn) 419.0 411.4 ± 2.3 kJ/mol 40.0639 ± 19527-08-3*12 Naphthalene cation [C6H4(C4H4)]+ (g) 956.72 933.65 ± 0.60 kJ/mol 128.1700 ± 34512-27-1*0 Naphthalene anion [C6H4(C4H4)]- (g) 187.4 164.5 ± 3.1 kJ/mol 128.1711 ± 34509-91-6*0 Isocyanic acid anion [HNCO]- (g) -54.3 -57.4 ± 3.0 kJ/mol 43.02533 ± *444010-28-0*0 COC vdW (CO)(C) (g) 589.7 598.5 ± 1.5 kJ/mol 40.0208 ± *12071-24-8*0 Dinitrogen anion [N2]- (g) 192.5 192.5 ± 1.7 kJ/mol 28.01403 ± 12185-02-3*0 Isocyanogen anion [CNCN]- (g) 386.6 388.7 ± 3.3 kJ/mol 52.0354 ± *83951-85-3*0 Cyanogen chloride anion [ClCN]- (g) 46.7 51.0 ± 3.1 kJ/mol 61.4707 ± 12281-43-5*0 Iminomethide [HCNH]- (g) 237.3 233.6 ± 1.8 kJ/mol 28.03387 ± 90623-30-6*0 Diphenylacetylene C6H5CCC6H5 (g) 407.6 ± 1.5 kJ/mol 178.2292 ± 501-65-5*0 Hydrodioxonitrate [HN(O)O]- (g) -61.8 -68.2 ± 3.2 kJ/mol 47.01403 ± 61508-71-2*0 3-Buten-2-one CH3C(O)CHCH2 (g, cis) -93.9 -109.9 ± 1.6 kJ/mol 70.0898 ± 78-94-4*2 Oxide [O]-2 (g) 651. 653. ± 15. kJ/mol 16.00050 ± 16833-27-5*0 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-trans, anti) -48.4 -64.7 ± 2.3 kJ/mol 70.0898 ± 59120-04-6*12 Cyanogen bromide cation [BrCN]+ (g) 1331.6 1325.3 ± 1.1 kJ/mol 105.9209 ± 34749-77-4*0 O--HH Complex (H2)(O) (g, vdW Cinfv ?) 247.6 248.9 ± 2.7 kJ/mol 18.01528 ± *7732-18-5*101 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-cis, anti) -38.3 -54.7 ± 2.3 kJ/mol 70.0898 ± 59120-04-6*22 Hydroxymethylene cation [HCOH]+ (g) 971.9 968.3 ± 1.4 kJ/mol 30.02543 ± 135395-06-1*0 Butadiynylium [CCCCH]+ (g) 1781.2 1787.7 ± 3.2 kJ/mol 49.0502 ± 76108-67-3*0 2-Iodoethanol ICH2CH2OH (g) -132.0 -150.2 ± 2.5 kJ/mol 171.9650 ± 624-76-0*0 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g, s-cis) -43.1 -59.1 ± 2.3 kJ/mol 70.0898 ± 59120-04-6*2 Diisocyanogen anion [CNNC]- (g) 456.8 462.5 ± 3.6 kJ/mol 52.0354 ± *78800-22-3*0 Diisocyanogen cation [CNNC]+ (g) 1825.3 1828.5 ± 3.2 kJ/mol 52.0343 ± *78800-21-2*0 1,3-Butadien-2-ol CH2C(OH)CHCH2 (g) -53.8 -68.7 ± 1.6 kJ/mol 70.0898 ± 59120-04-6*0 Water H2O (cr, l, eq.press.) -286.300 -285.828 ± 0.026 kJ/mol 18.01528 ± 7732-18-5*499 Fluorine atom F (g, 2P1/2) 82.093 83.878 ± 0.047 kJ/mol 18.99840320 ± 14762-94-8*2 Dinitrogen tetraoxide O2NNO2 (cr,l) -37.84 -26.99 ± 0.19 kJ/mol 92.0111 ± 10544-72-6*500 Difluoromethane CH2F2 (l) -468.74 ± 0.36 kJ/mol 52.02339 ± 75-10-5*590 Chlorous acid anion [HOClO]- (g) -154.2 -156.1 ± 3.3 kJ/mol 68.4600 ± 99559-66-7*0 Hydroperoxonium [H2OOH]+ (g) 738.1 727.8 ± 1.2 kJ/mol 35.02207 ± 70850-26-9*0 Chlorine pentafluoride cation [FCl(F)(F)(F)F]+ (g) 1005.8 996.8 ± 3.3 kJ/mol 130.44417 ± 478167-78-1*0 Methylaminyliumyl [CH3N]+ (g, quartet) 1423.0 1416.1 ± 2.3 kJ/mol 29.04071 ± 56924-51-7*2 1,2-Propadien-1-one anion [CH2CCO]- (g) 49.5 48.3 ± 3.1 kJ/mol 54.0479 ± 159171-62-7*0 Trioxodinitrate anion [ONN(O)O]- (g) -104.2 -107.5 ± 1.9 kJ/mol 76.01223 ± 194856-62-7*0 Krypton dication [Kr]+2 (g) 3701.124 3701.124 ± 0.013 kJ/mol 83.7989 ± 18469-91-5*0 Propargylenide [HCCCH]- (g) 434.4 437.6 ± 1.1 kJ/mol 38.0485 ± 82906-05-6*0 Xenon dication [Xe]+2 (g) 3194.14 3194.14 ± 0.36 kJ/mol 131.2889 ± 14066-95-6*0 Carbon C (g, quintuplet) 1114.961 1120.107 ± 0.045 kJ/mol 12.01070 ± 7440-44-0*3 Neon dication [Ne]+2 (g) 6032.988 6033.214 ± 0.0041 kJ/mol 20.17860 ± 14041-55-5*0 Neon trication [Ne]+3 (g) 12152.403 12152.403 ± 0.033 kJ/mol 20.17805 ± 14158-25-9*0 Neon tetracation [Ne]+4 (g) 21529.82 21531.45 ± 0.25 kJ/mol 20.17751 ± 14041-56-6*0 Neon pentacation [Ne]+5 (g) 33711.1 33711.1 ± 1.2 kJ/mol 20.17696 ± 14175-48-5*0 Neon hexacation [Ne]+6 (g) 48949.3 48949.3 ± 1.3 kJ/mol 20.17641 ± 14041-57-7*0 Neon heptacation [Ne]+7 (g) 68948.7 68948.7 ± 1.5 kJ/mol 20.17586 ± 14175-50-9*0 Neon octacation [Ne]+8 (g) 92018.2 92018.2 ± 1.5 kJ/mol 20.17531 ± 14782-26-4*0 Neon nonacation [Ne]+9 (g) 207396.1 207396.1 ± 1.5 kJ/mol 20.17476 ± 15721-59-2*0 Neon cation Ne+ (g) 2080.662 2080.769 ± 0.000 kJ/mol 20.17915 ± 14782-23-1*0 Neon decacation [Ne]+10 (g) 338828.3 338828.3 ± 1.5 kJ/mol 20.17421 ± 32218-07-8*0 Argon dimer cation [Ar2]+ (g) 1393.760 1391.173 ± 0.018 kJ/mol 79.8955 ± 17596-58-6*0 Argon cation Ar+ (g) 1520.571 1520.580 ± 0.000 kJ/mol 39.94745 ± 14791-69-6*0 Argon dimer Ar2 (g) -1.013 -6.347 ± 0.0051 kJ/mol 79.8960 ± 12595-59-4*0 Helium cation He+ (g) 2372.322 2372.322 ± 0.000 kJ/mol 4.0020534 ± 14234-48-1*0 Helium dication [He]+2 (g) 7622.839 7622.839 ± 0.000 kJ/mol 4.0015048 ± 12587-46-1*0 Helium anion He- (g, 1s2.2s 2S1/2) 51. 51. ± 130. kJ/mol 4.0031506 ± 14452-58-5*1 Helium anion He- (g) 51. 51. ± 130. kJ/mol 4.0031506 ± 14452-58-5*0 Argon anion Ar- (g, 2S1/2) 23. 23. ± 75. kJ/mol 39.94855 ± 16544-90-4*1 Argon anion Ar- (g) 23. 23. ± 75. kJ/mol 39.94855 ± 16544-90-4*0 Helium anion He- (g, 1s.2s2 2S1/2) 1868.43 1868.43 ± 0.11 kJ/mol 4.0031506 ± 14452-58-5*2 Helium anion He- (g, 1s.2s.2p 4P) 1904.823 1904.824 ± 0.0011 kJ/mol 4.0031506 ± 14452-58-5*3 Helium anion He- (g, 2p3 4S3/2) 5724.9 5724.9 ± 1.5 kJ/mol 4.0031506 ± 14452-58-5*4 Argon anion Ar- (g, 4S3/2) 1111.116 1111.116 ± 0.098 kJ/mol 39.94855 ± 16544-90-4*2 Hydron H+ (aq) 0 0 exact 1.007391 ± 12408-02-5*800
References
1
B. Ruscic, R. E. Pinzon, M. L. Morton, G. von Laszewski, S. Bittner, S. G. Nijsure, K. A. Amin, M. Minkoff, and A. F. Wagner, J. Phys. Chem. A 108 , 9979-9997 (2004)
[DOI: 10.1021/jp047912y]
2
B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, st Century. J. Phys. Conf. Ser. 16 , 561-570 (2005)
[DOI: 10.1088/1742-6596/16/1/078]
3
B. Ruscic and D. H. Bross, [DOI: 10.17038/CSE/1822363] ; available at ATcT.anl.gov
4
D. Feller, D. H. Bross, and B. Ruscic, J. Phys. Chem. A 123 , 3481-3496 (2019)
[DOI: 10.1021/acs.jpca.8b12329]
5
B. K. Welch, R. Dawes, D. H. Bross, and B. Ruscic, J. Phys. Chem. A 123 , 5673-5682 (2019)
[DOI: 10.1021/acs.jpca.8b12329]
6
D. H. Bross, H.-G. Yu, L. B. Harding, and B. Ruscic, J. Phys. Chem. A 123 , 4212-4231 (2019)
[DOI: 10.1021/acs.jpca.9b02295]
7
B. Ruscic, Int. J. Quantum Chem. 114 , 1097-1101 (2014)
[DOI: 10.1002/qua.24605]
Formula
The aggregate state is given in parentheses following the formula, such as: g - gas-phase, cr - crystal, l - liquid, etc.
Uncertainties
The listed uncertainties correspond to estimated 95% confidence limits, as customary in thermochemistry (see, for example, Ruscic [7 ]). 5 kJ/mol.
Website Functionality Credits
The reorganization of the website was developed and implemented by David H. Bross (ANL). Indigo-depict . Jmol: an open-source Java viewer for chemical structures in 3D. http://www.jmol.org/ .
Acknowledgement
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. DE-AC02-06CH11357.








![[H][H]](images/1.png)
![[He]](images/2.png)
![[C]](images/3.png)



![[Ne]](images/7.png)
![[Si]](images/8.png)

![[Ar]](images/10.png)

![[Kr]](images/12.png)

![[Xe]](images/14.png)
![[2H][2H]](images/15.png)
![[H]](images/16.png)
![[C]](images/17.png)
![[O]](images/18.png)
![[N]](images/19.png)
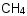
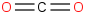

![[Cl]](images/23.png)

![[H+]](images/25.png)

![[F]](images/27.png)

![[CH3]](images/29.png)

![[Br]](images/31.png)

![[OH]](images/33.png)

![[C-]#[O+]](images/35.png)
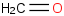
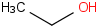




![[I]](images/42.png)
![C[CH2]](images/43.png)

![[N]=O](images/45.png)
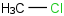
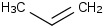
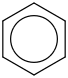

![O=[N]=O](images/50.png)

![[OH-]](images/52.png)
![O[O]](images/53.png)
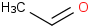
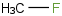

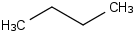
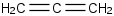
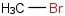
![[Si]](images/60.png)

![[CH2]](images/62.png)
![[CH2]](images/63.png)
![C=[CH]](images/64.png)
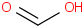
![C[O]](images/66.png)




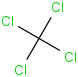
![[F-]](images/72.png)

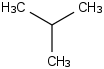
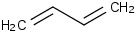
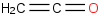
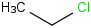
![c1cccc[c]1](images/78.png)
![[CH2]O](images/79.png)
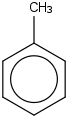
![[CH]](images/81.png)
![C[O-]](images/82.png)
![[C]#N](images/83.png)

![[SiH4]](images/85.png)
![[CH2]C#C](images/86.png)
![O=[O+][O-]](images/87.png)

![[OH3+]](images/89.png)

(F)F](images/91.png)
![[CH2+]1[CH2][H]1](images/92.png)
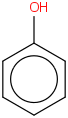
![[Cl-]](images/94.png)
![[Br-]](images/95.png)
![[CH+]=O](images/96.png)

![[NH2]](images/98.png)
![[CH]=O](images/99.png)

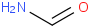

![C=C[CH2]](images/103.png)
![[NH]](images/104.png)
![[O-]\[O+]=C](images/105.png)

[O-]](images/107.png)
![CC[O-]](images/108.png)
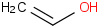
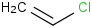
![[CH3+]](images/111.png)
![[I-]](images/112.png)

![CO[O]](images/114.png)
![O[C]=O](images/115.png)
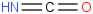

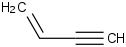




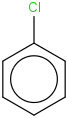
![C#[C]](images/124.png)
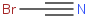
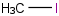
![[O-]](images/127.png)
![CC[CH2]](images/128.png)
![C[CH]C](images/129.png)
![[H]1[H+][H]1](images/130.png)
![C[C]=O](images/131.png)

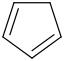
![[CH2]CO](images/134.png)
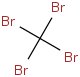

![C#[C-]](images/137.png)

![[Cl-]=O](images/139.png)


![C[N+]#[C-]](images/142.png)

![[CH]=C=[CH]](images/144.png)



![[C]F](images/148.png)
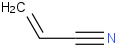
![[SiH3]O](images/150.png)
![[O+]](images/151.png)

![[C]=C=[C]](images/153.png)

![C[CH]O](images/155.png)
![CC[O]](images/156.png)
![C(=O)[O-]](images/157.png)




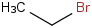
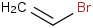
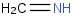


![N#[NH+]](images/167.png)
![O=[N-]=O](images/168.png)

![[H-]](images/170.png)
![CCC[CH2]](images/171.png)


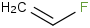
![c1cccc[c+]1](images/175.png)
![[O]F](images/176.png)

![[CH5+]](images/178.png)

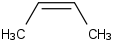
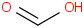
![N#C[O]](images/182.png)
![[N]=C=[N]](images/183.png)
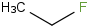
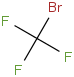
![c1cccc[c-]1](images/186.png)
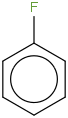
[O-]](images/188.png)
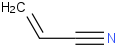
![C=C=C=[CH]](images/190.png)

![O=[O+]](images/192.png)
![[C]=[C]](images/193.png)
![[CH]](images/194.png)
![C[CH2-]](images/195.png)
![[CH+]1[CH][H]1](images/196.png)
![C=[CH-]](images/197.png)
![[CH2]N](images/198.png)
![[C-]#N](images/199.png)
![[CH2]C#N](images/200.png)

![C=C=[C]](images/202.png)
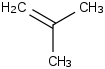
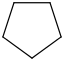
![c1ccc(cc1)[O-]](images/205.png)
![[CH]1C=CC=C1](images/206.png)
![N[C]=O](images/207.png)
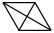


![Br[O]](images/211.png)
![Br[O-]](images/212.png)
![[NH4+]](images/213.png)
![[H]/N=N/[H]](images/214.png)

![[CH]C](images/216.png)

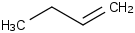

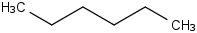

Cl](images/222.png)
(F)F](images/223.png)



![[N]N=[N]](images/227.png)
![[N-]=[N+]=O](images/228.png)
![O[C+]=O](images/229.png)
![[NH]O](images/230.png)
![N=[N+]=[N-]](images/231.png)
![[CH]C](images/232.png)
![[C]=C](images/233.png)
![C[C+]=O](images/234.png)
![[CH2+]O](images/235.png)

![C(=O)O[O]](images/237.png)
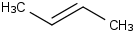
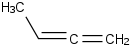

![[CH]=C=O](images/241.png)
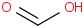
![O[O-]](images/243.png)


(Cl)Cl](images/246.png)


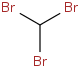
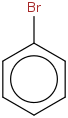
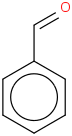
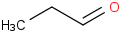

![C#C[N+]#[C-]](images/254.png)
![[SiH2]=O](images/255.png)
![[SiH3][O]](images/256.png)

![I[O]](images/258.png)
![[C]#[O+]](images/259.png)
![[NH2-]](images/260.png)

![N[O]](images/262.png)
[O-]](images/263.png)
![FO[O]](images/264.png)

![[CH-]=O](images/266.png)
![C[C]=C](images/267.png)
![C=C=[CH-]](images/268.png)
![C#C[C]](images/269.png)
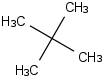
![[O-]F](images/271.png)
![CC[O]](images/272.png)
![[C]=C=O](images/273.png)
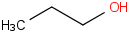
![[CH]Cl](images/275.png)



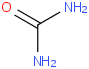
![[NH2+]=C=O](images/280.png)
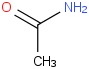

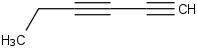

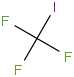
![[N]N=[N+]](images/286.png)
![N#[O-]](images/287.png)
![N#[O+]](images/288.png)
[O]](images/289.png)
![[CH2-]](images/290.png)
![[CH-]](images/291.png)
![[CH3+][H][CH3]](images/292.png)
![[C]=N](images/293.png)
![[CH]O](images/294.png)
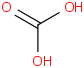
![C(=O)O[O-]](images/296.png)
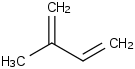
![C[CH+]O](images/298.png)
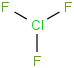

![C(=O)[O]](images/301.png)
![[H][ClH+]](images/302.png)

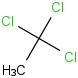

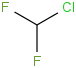
![[CH]F](images/307.png)
![F[C]=O](images/308.png)
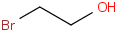
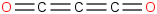
![c1ccc(cc1)[CH2]](images/311.png)
![c1ccc(cc1)[O]](images/312.png)
![C=[C]C#N](images/313.png)
![[CH]C#N](images/314.png)
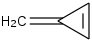
![c1ccc(cc1)O[O]](images/316.png)
C](images/317.png)

![O[OH+]](images/319.png)
![[H][BrH+]](images/320.png)
![[CH3-]](images/321.png)
![[CH2+]](images/322.png)
![[C]C](images/323.png)
![[CH2+]N](images/324.png)
![[CH2-][N+]#[C-]](images/325.png)
![C[C]C](images/326.png)
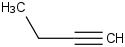
![OO[O-]](images/328.png)
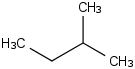
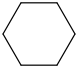
![C[CH]O](images/331.png)
![[OH+]1CC1](images/332.png)



![C#[N+][O-]](images/336.png)
![O[N+]#[C-]](images/337.png)
![[C]N=O](images/338.png)


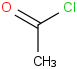
O](images/342.png)

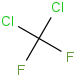

F](images/346.png)
![[CH]F](images/347.png)
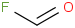
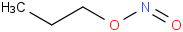
![[CH]=CC#N](images/350.png)
![C=C=C=[CH+]](images/351.png)
![C#CC=[CH]](images/352.png)
![[SiH3]](images/353.png)
![[SiH2]O](images/354.png)
(C)O](images/355.png)

![I[O-]](images/357.png)
![N#[N+]](images/358.png)
=O](images/359.png)
![[NH4+]](images/360.png)
![N[NH]](images/361.png)
![C[NH]](images/362.png)
![C=[N]](images/363.png)
![[CH]=N](images/364.png)
![[C+]=N](images/365.png)

![Cl[Cl-]](images/367.png)
![C[CH+]C](images/368.png)
![[Cl+]](images/369.png)
![CC[CH2-]](images/370.png)
![[OH2+]O](images/371.png)
![CC=[CH]](images/372.png)
![C#C[CH-]](images/373.png)
C](images/374.png)


![CC#C[CH2]](images/377.png)
![[CH2]CO](images/378.png)
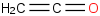

![[CH]C=O](images/381.png)



![[CH2]C(=O)O](images/385.png)
![CN[CH2]](images/386.png)
![C[N]C](images/387.png)
![[NH]C#N](images/388.png)
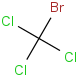
![[C]Cl](images/390.png)
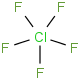

![C(Cl)[O]](images/393.png)

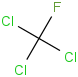

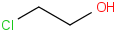
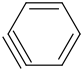
![[CH]1C=CC=CC=C1](images/399.png)
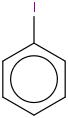
![CC(=O)[CH2]](images/401.png)

![C=C[N+]#[C-]](images/403.png)
![C[C][N+]#[C-]](images/404.png)
![C=C1C=[C]1](images/405.png)

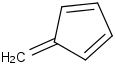
![[Si]=O](images/408.png)
![[SiH3][O-]](images/409.png)
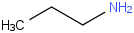
![O=Cl(=O)[O]](images/411.png)

[O-]](images/413.png)
![[N]N](images/414.png)
![[NH+]=O](images/415.png)
[O-]](images/416.png)

![[C-]](images/418.png)
![O=Cl(=O)[O-]](images/419.png)
![[C]C](images/420.png)
![C#[C+]](images/421.png)
![C[NH-]](images/422.png)
![[NH+]#C](images/423.png)

![CO[O-]](images/425.png)
![CC[C]](images/426.png)

![[C]1CC1](images/428.png)
![[CH2+]C#C](images/429.png)
![C1C=[C]1](images/430.png)
![C#C[CH-]](images/431.png)
![C#C[C+]](images/432.png)
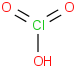
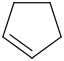

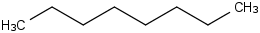
![F[F-]](images/437.png)
![[O][OH][O]](images/438.png)
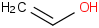
![C=C[O]](images/440.png)
![C[C-]=O](images/441.png)

![O[C]=O](images/443.png)
O](images/444.png)


![CC(=O)[O-]](images/447.png)
![CN(C)[CH2]](images/448.png)
![[CH2-]Cl](images/449.png)

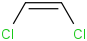
![[Br+]](images/452.png)

F](images/454.png)


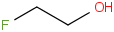
![[H][F+][H]](images/458.png)
![c1ccc(cc1)[CH2-]](images/459.png)
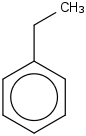
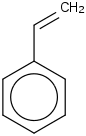
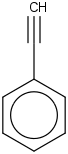
![[N+]](images/463.png)
![CC(=[O-])[CH2]](images/464.png)

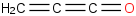
![C[C]C#N](images/467.png)
![[CH]C=C=N](images/468.png)
![[CH]=CN=[CH]](images/469.png)
![C=[C-]C#N](images/470.png)
![[CH]=CC#N](images/471.png)
![C=CC#[C]](images/472.png)

![C[SiH3]](images/474.png)
(C)[O]](images/475.png)
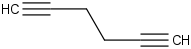
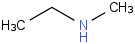
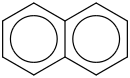

![[C+]](images/480.png)
![[CH+]](images/481.png)
![[C]C](images/482.png)
![[C-]=C](images/483.png)
![O=[O-]=O](images/484.png)

![[OH+]](images/486.png)
![[CH2-]C#N](images/487.png)
![[N]1OO1](images/488.png)
![[CH3+]O](images/489.png)
![CO.[H+]](images/490.png)
![[CH]O](images/491.png)
![[C].O](images/492.png)
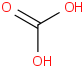
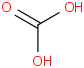
[O-]](images/495.png)
![CC[CH2+]](images/496.png)
![CC[CH]](images/497.png)
![C=C[CH2-]](images/498.png)
![C1C[CH]1](images/499.png)
![CC[C]](images/500.png)
![C1=C[CH+]1](images/501.png)
![CC#[C-]](images/502.png)
![[CH]C=[CH]](images/503.png)
![CC[CH]C](images/504.png)
C](images/505.png)

![OO[O]](images/507.png)

![C1[C]O1](images/509.png)

O](images/511.png)

![[O]](images/513.png)

![CO[C]=O](images/515.png)
![[OH-]](images/516.png)
![O=CO[CH2]](images/517.png)

![C(=O)[C]=O](images/519.png)

![CN[CH2+]](images/521.png)
![[CH]=[N]=[N]](images/522.png)
![[NH][N+]#[C-]](images/523.png)

Cl](images/525.png)
![[CH2]Cl](images/526.png)



F](images/530.png)
![[C+]F](images/531.png)

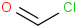
![Cl[C]=O](images/534.png)
![C1=CC#CC=[CH-]1](images/535.png)
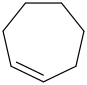
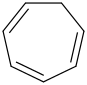
![c1ccc(cc1)[F+]](images/538.png)
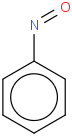
[O-]](images/540.png)
[O]](images/541.png)
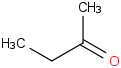
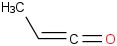
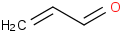

![O=[O-]](images/546.png)
![[C]1=CC=C1](images/547.png)
![[NH3+]](images/548.png)
![[SiH2]](images/549.png)
(C)[O-]](images/550.png)

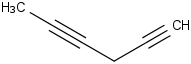
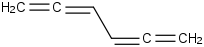
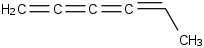
![C/C=C/[CH2]](images/555.png)

![[C]#CC#C](images/557.png)
([O-])OO](images/558.png)

![F.[F]](images/560.png)
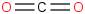
![C12=[C]C2=[C]1](images/562.png)
![[CH4+]](images/563.png)
![[O]](images/564.png)
![[H][O+][H]](images/565.png)
![[CH3+][H][CH3]](images/566.png)
![C#[CH+]](images/567.png)
![[C+]C](images/568.png)
![[C-]C](images/569.png)
![[H][Cl](=O)=O](images/570.png)
![F.[F-]](images/571.png)
![[CH2][N+]#[C-]](images/572.png)
![C[O+]](images/573.png)
![[CH]O](images/574.png)
![[N]1N=[N]1](images/575.png)
![[CH+]O](images/576.png)
F](images/577.png)
![[C]O](images/578.png)
![OO[O+]](images/579.png)
![C[C]C](images/580.png)
![C=C[CH2+]](images/581.png)
![CC=[CH]](images/582.png)
![[CH]1C#C1](images/583.png)

![CCC[CH2-]](images/585.png)
![CC[CH-]C](images/586.png)
![C[CH+]O](images/587.png)
![[I+]](images/588.png)
![CO[CH2]](images/589.png)
![C=C[OH+]](images/590.png)
![C1[CH]O1](images/591.png)
![C1[CH+]O1](images/592.png)
![[H][O-][H]](images/593.png)
![[C]=[O]=[C]](images/594.png)
![[C]=[O]=[C]](images/595.png)
![[CH]C=O](images/596.png)

O](images/598.png)
![O[O+]](images/599.png)
![C(=O)[O]](images/600.png)
![[N]=N](images/601.png)
![O=CO[CH2]](images/602.png)
![[CH2]C(=O)O](images/603.png)
![C[N-]C](images/604.png)



Cl](images/608.png)
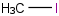
F](images/610.png)
![[Br-]](images/611.png)


![[C]#CF](images/614.png)
![F[C]OF](images/615.png)
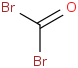
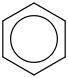
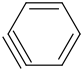
![c1[c]ccc[c]1](images/619.png)
![c1c[c]cc[c]1](images/620.png)
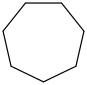
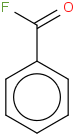
![[F-]](images/623.png)
![[N]O](images/624.png)
O[N+](=O)[O-]](images/625.png)
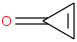
![[I-]](images/627.png)
![[C]=CC#N](images/628.png)
![[C]=C[N+]#[C-]](images/629.png)
![[C-]#CC#N](images/630.png)
![C=C[N+]#[C-]](images/631.png)
![C[C]C#N](images/632.png)
![[CH][N+]#[C-]](images/633.png)
![CC1=C[C]1](images/634.png)
![C=C=C=[CH+]](images/635.png)
![C=C=C=[CH-]](images/636.png)
![C#CC=[CH-]](images/637.png)
![[N]N=[N-]](images/638.png)
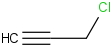
![O=[Si]=O](images/640.png)
![[SiH2]](images/641.png)
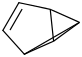
![[CH]](images/643.png)
![[F+]F[H]](images/644.png)
![C[NH3+]](images/645.png)
![[CH][NH2]](images/646.png)
![C[N]](images/647.png)
![C=[N-]](images/648.png)
![[CH]=N](images/649.png)
![[C]N](images/650.png)
![F.[F-]](images/651.png)
![[CH2].O](images/652.png)

![[CH-]O](images/654.png)
![[C+]O](images/655.png)
![[C][O].[H-]](images/656.png)

[O]](images/658.png)
![OO[C]=O](images/659.png)
F](images/660.png)
![[CH2]C[CH]](images/661.png)
![CC=[C]](images/662.png)
![O=[F+]](images/663.png)
![CC#[C]](images/664.png)
![C1=C[CH]1](images/665.png)
![[CH2]C=[C]](images/666.png)
![[H]/N=[N+]/[H]](images/667.png)

![C#C[C-]](images/669.png)
C](images/670.png)
![[H]/N=N\[H]](images/671.png)
![CC(C)[CH2]](images/672.png)
![[2H]](images/673.png)
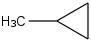

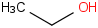

![[NH-][O+]=O](images/678.png)
![O[O+]O](images/679.png)
![[CH2]CO](images/680.png)
![CC[O]](images/681.png)
![[H]O[H][O+]](images/682.png)
![C=C[OH+]](images/683.png)
![[CH2+]C=O](images/684.png)
![[CH2-]C=O](images/685.png)
![[CH]=CO](images/686.png)
![C1[CH-]O1](images/687.png)

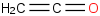
![[C]=O.[C]](images/690.png)

![[C]1OO1](images/692.png)
![[Xe+]](images/693.png)
![C(=O)[O+]](images/694.png)
![[CH]O[O]](images/695.png)

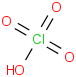
![N#C[O-]](images/698.png)
![[C]1=NO1](images/699.png)

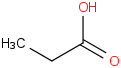
![[CH2]C(=O)OC](images/702.png)
![CC(=O)O[CH2]](images/703.png)
![[Cl+]F](images/704.png)
[CH2]](images/705.png)
(Cl)Cl](images/706.png)
![[O][OH][O+]](images/707.png)
![[N+]O](images/708.png)





![[H]O[H][O-]](images/714.png)
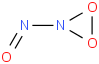

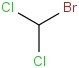
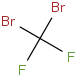
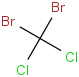
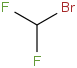
![[O][OH][O-]](images/721.png)

![F[C]OF](images/723.png)
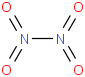
![ClO[Cl]=O](images/725.png)
![c1cccc[c+]1](images/726.png)
![[NH]O](images/727.png)
![[NH+]O](images/728.png)
![c1ccc(cc1)[Cl+]](images/729.png)
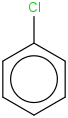
![c1(Cl)c[c]ccc1](images/731.png)
![c1(Cl)cc[c]cc1](images/732.png)
![c1(Cl)[c]cccc1](images/733.png)
![c1ccc(cc1)[Br+]](images/734.png)
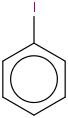
[O-]](images/736.png)

![[CH2]N[O]](images/738.png)
![[OH2][O]](images/739.png)
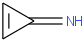
![C=[C][N+]#[C-]](images/741.png)
![[H]Cl[O]](images/742.png)
![[Cl+]O([H])[H]](images/743.png)
![I[I-]I](images/744.png)
![C=CC=[C]](images/745.png)
![C#CC=[CH-]](images/746.png)
![[C-]12C3C1C23](images/747.png)
![[Si]=[C]](images/748.png)
![[Si]=[C]](images/749.png)
(F)(F)F](images/750.png)
![[SiH3][SiH3]](images/751.png)
C](images/752.png)
![C[SiH2]C](images/753.png)
![FCl[H+]](images/754.png)
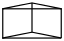
![F.[H-]](images/756.png)
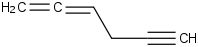
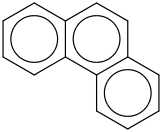
![[C-]#CC#C](images/759.png)
![ClF[H+]](images/760.png)
![IC#[N-]](images/761.png)
![[NH4+].O=[N+]([O-])[O-]](images/762.png)

![C=[O+]](images/764.png)
![O=ClO[O-]](images/765.png)
![[C][O].[H-]](images/766.png)
![[Cl-]F](images/767.png)

![[OH][H][OH-]](images/769.png)

![C=C=[CH2+]](images/771.png)
![[N]O[O]](images/772.png)
![C=[CH2+]](images/773.png)
![[CH]C=C](images/774.png)
![[CH]C=C](images/775.png)
![C1C=[C-]1](images/776.png)
(F)F](images/777.png)
![C1=[C+]=C1](images/778.png)
![[CH]=C=[CH]](images/779.png)
![[CH][CH][C]](images/780.png)
![CCC[CH2+]](images/781.png)
![I[I-]](images/782.png)

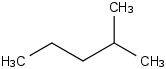
![[N]O[N]](images/785.png)
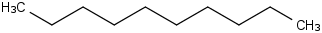
![CC[OH+]](images/787.png)
![C[CH+]O](images/788.png)
![[CH2+]CO](images/789.png)
![CC[O+]](images/790.png)
![CC=[O+]](images/791.png)
![[N]=NN=[N]](images/792.png)
![C1[CH+]O1](images/793.png)
![C1[CH-]O1](images/794.png)
![[I]](images/795.png)
![[CH-]=C=O](images/796.png)
![[C-]=C=O](images/797.png)
![[C]=C=O](images/798.png)
![[C]=O.[C]](images/799.png)
![[C]=[O+][C]](images/800.png)
![[C-]=[O+]=[C-]](images/801.png)
![C1=C[O+]1](images/802.png)

![[CH]C=O](images/804.png)
![[C]=C](images/805.png)
![[CH2]OO](images/806.png)
O](images/807.png)
O](images/808.png)
![[C]O[O]](images/809.png)
![[NH+]=C=O](images/810.png)
![C#[N+][O]](images/811.png)

![I[I-]I](images/813.png)
![Br[Br-]](images/814.png)
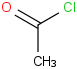


![N#N.[H-]](images/818.png)
![[CH2]C(=O)OC](images/819.png)
![CC(=O)O[CH2]](images/820.png)
![O[C]=C=O](images/821.png)
![O=[N+]=O](images/822.png)
![[C][N+]#[C-]](images/823.png)
![[C]C#N](images/824.png)
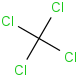
![[Cl-]](images/826.png)
Cl](images/827.png)
Cl](images/828.png)
Cl](images/829.png)
![[CH2+]Cl](images/830.png)
![[CH]Cl](images/831.png)


![ClCl[O]](images/834.png)
![F.[F]](images/835.png)

![[C]=[C-]](images/837.png)

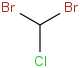
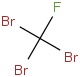
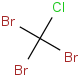
![O.O=[O+]](images/842.png)


![[CH]F](images/845.png)
![[CH-]=N](images/846.png)


![F[C]OF](images/849.png)
![F[C+]=O](images/850.png)
![[C]OF](images/851.png)
![c1ccc(cc1)[H+]](images/852.png)

![[C+]1=C=C=1](images/854.png)
![[H][F+][H]](images/855.png)
![ClOO[O]](images/856.png)
![c1(Cl)c[c-]ccc1](images/857.png)
![c1(Cl)cc[c-]cc1](images/858.png)
![c1(Cl)[c-]cccc1](images/859.png)
![[H][F+][H]](images/860.png)
![CC(=[O+])[CH2]](images/861.png)


![[Cl+]=O](images/864.png)
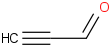
![FF[O]](images/866.png)
![[C]#CC#[C]](images/867.png)
![[F-].[H][H]](images/868.png)
![C[C][N+]#[C-]](images/869.png)
![[CH]C=C=N](images/870.png)
![[CH]=CN=[CH]](images/871.png)

![[CH+]=CC#N](images/873.png)
![[CH-]=CC#N](images/874.png)
![[CH]=C[N+]#[C-]](images/875.png)
![[C]=C=C=O](images/876.png)

![C=CC#[CH+]](images/878.png)
![[C+]12=CC2C1](images/879.png)
[O-]](images/880.png)

![[CH]=C1C=C1](images/882.png)
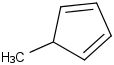
![[Si][Si]](images/884.png)
![[SiH3]O[SiH3]](images/885.png)
(C)C](images/886.png)
C](images/887.png)
![[SiH2-]O](images/888.png)
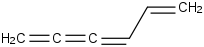
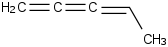

![[NH2+]](images/892.png)
![[N]O[O-]](images/893.png)
F](images/894.png)
![C=[CH2-]](images/895.png)
![OO[O+]](images/896.png)
![C#C[CH+]](images/897.png)
![N#[N+]=O](images/898.png)
![[CH]=C=[CH]](images/899.png)
![[Cl]FF](images/900.png)
![C=C=[C-]](images/901.png)
![C=C=[C+]](images/902.png)
![[H]/N=[N-]/[H]](images/903.png)

![FO[O+]](images/905.png)
![CC#C[CH2-]](images/906.png)
![[H]/N=[N+]\[H]](images/907.png)
![C=C[C]=C](images/908.png)
![[F-]O[O]](images/909.png)

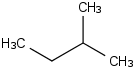
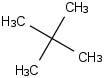
![[O]F[O]](images/914.png)
![[N-]1=NO1](images/917.png)
![C[CH3+]](images/919.png)
![[CH2+].O](images/920.png)
![O=Cl(=O)(=O)[O]](images/921.png)
![O=Cl(=O)(=O)[O-]](images/922.png)
![[CH2-]O](images/923.png)
[O-]](images/924.png)
![C#[CH-]](images/925.png)
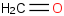
[O-]](images/927.png)
![[C+]=C=O](images/928.png)
![ClOO[O-]](images/929.png)
![[N]=NN=[N]](images/930.png)
![[C]=[O+][C]](images/931.png)
![C1=C[O-]1](images/932.png)
![[CH+]O](images/933.png)
![[CH+]O](images/934.png)
![[CH]C=O](images/935.png)
![[CH-]O](images/936.png)
![[CH-]O](images/937.png)
O](images/938.png)
![[N+]N](images/939.png)
![C1[O+]O1](images/940.png)
![C=[O+][O]](images/941.png)
![[H][H+]](images/942.png)
![[CH]O[O]](images/943.png)
![[O][H].[C]#O](images/944.png)
![[O][H].O#[C]](images/945.png)
![N#C[O+]](images/946.png)
![[C+]N=O](images/947.png)
![[C-]#[N+][O-]](images/948.png)



![[C].O](images/952.png)
![[C+].O](images/953.png)

![N#N.[H-]](images/955.png)



![ClOO[O-]](images/959.png)

![C[N+]C](images/961.png)
![[C-]#[N+][N+]#[C-]](images/962.png)


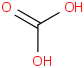
Cl](images/966.png)
![N[OH+]](images/967.png)
[O-]](images/968.png)

![[CH+]Cl](images/970.png)
![[CH-]Cl](images/971.png)
O](images/972.png)


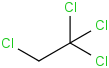

![[C]=[C+]](images/977.png)
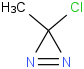

![O[OH+]](images/980.png)
![O[O+]O](images/981.png)
![FF[F-]](images/982.png)
![F[H][F-]](images/983.png)
![[N+]1OO1](images/984.png)
![[NH+]](images/985.png)

![[NH3+][O-]](images/987.png)

![[CH+]F](images/989.png)
![[CH-]F](images/990.png)
![FC(C(F)(F)[*:1])(F)[*:2]](images/991.png)
![[FH+]](images/992.png)
![[FH-]](images/993.png)

![N[O+]](images/995.png)
![[C]#C[F-]](images/996.png)
![[CH-]=N](images/997.png)
![F[CH+]=O](images/998.png)
![F[C-]=O](images/999.png)
![[C+]OF](images/1000.png)
![[2H+]](images/1001.png)
![O=ClO[O]](images/1002.png)
![C1=CC#CC=[CH+]1](images/1003.png)

![c1[c]ccc[c+]1](images/1005.png)
![c1c[c]cc[c]1](images/1006.png)
![c1ccc(cc1)[CH2+]](images/1007.png)
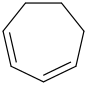
![[CH+]1C=CC=CC=C1](images/1009.png)
![[NH+]O](images/1010.png)
![[CH+]#N](images/1011.png)
![[NH-]O](images/1012.png)

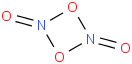
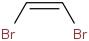

![[C+]#N](images/1017.png)
![OO[O]](images/1018.png)
![N=[C]O](images/1019.png)
![[CH2]N=O](images/1020.png)
![[CH]=NO](images/1021.png)

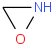
![[C]#CC#N](images/1024.png)
![[OH2][O+]](images/1025.png)
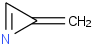
![[CH+]=CC#N](images/1027.png)
![[CH-]=CC#N](images/1028.png)
![[CH]=C[N+]#[C-]](images/1029.png)
![[CH]C#N](images/1030.png)
![[CH][N+]#[C-]](images/1031.png)
![[N-]1OO1](images/1032.png)
![[CH2+]=C1C=C1](images/1033.png)
![O=[Cl-]=O](images/1034.png)
![CC1=[C][CH+]1](images/1035.png)
![C=CC=[C+]](images/1036.png)
![CC=C=[C]](images/1037.png)
![F.[H-]](images/1038.png)

![C#CC=[CH+]](images/1040.png)
![CC#[CH+]](images/1041.png)
![C=CC#[C+]](images/1042.png)
![C=C1C=[C+]1](images/1043.png)
![C=C1C=[C-]1](images/1044.png)
![C1[CH]C=C1](images/1045.png)
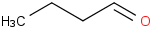
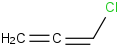

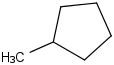
![O=[Si]=O](images/1050.png)
![O=[Si]=O](images/1051.png)
![[Si][C+]](images/1052.png)
![[SiH3-]](images/1053.png)
![[SiH2+]](images/1054.png)
![FF[O-]](images/1055.png)

![OOO[O]](images/1057.png)
![[N]O[O+]](images/1058.png)

![[C]1CC1](images/1060.png)

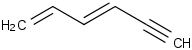
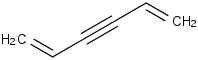
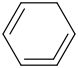
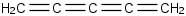
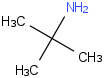
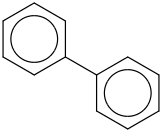
![[Kr+][H]](images/1068.png)
![C1[C]=[C]1](images/1069.png)
![C1[C]O1](images/1070.png)
![C1[C+]O1](images/1071.png)
![C1[C-]O1](images/1072.png)
![[N-]=[N]=O](images/1073.png)
![C[O+]O](images/1074.png)
![CO[O+]](images/1075.png)
![[CH-]C](images/1076.png)
![[CH+]1C#C1](images/1077.png)
![[ClH+]](images/1078.png)
![[CH-]1C#C1](images/1079.png)
![C1[O-]O1](images/1080.png)
![C=[O][O-]](images/1081.png)
![[N]=NN=[N+]](images/1082.png)
![C[NH2+]](images/1083.png)
![[CH3-]O](images/1084.png)
![[H].O=C=O](images/1085.png)
![C[NH2-]](images/1086.png)

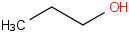
[O-]](images/1089.png)
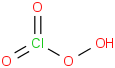
![[C+]1=NO1](images/1091.png)
![[C-]1=NO1](images/1092.png)
![[Cl+]FF](images/1093.png)
![[Cl-]FF](images/1094.png)
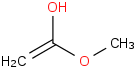
![[F+]F[H]](images/1096.png)
![[CH2-]N](images/1097.png)

![[CH3+][H][CH3]](images/1099.png)
![I[I+]I](images/1100.png)
![[CH3+][H][CH3]](images/1101.png)
![C[NH+]](images/1102.png)
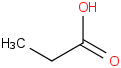


![C=[NH+]](images/1106.png)
![N#C[C+]#N](images/1107.png)
![[C-]#[N+]C#N](images/1108.png)
![[N-]=[N+]=C=[C]](images/1109.png)
![[NH4+].[Br-]](images/1110.png)
![[C][N+]#[C]](images/1111.png)
![[C][N]#[C-]](images/1112.png)
![[C+]C#N](images/1113.png)
![[C-]C#N](images/1114.png)
![[N]1C#C1](images/1115.png)

![[NH+]C#N](images/1117.png)
![[NH-]C#N](images/1118.png)

![[C]=N=[N]](images/1120.png)

(Cl)Cl](images/1122.png)
![C=[NH-]](images/1123.png)
Cl](images/1124.png)

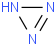

![C=C[C-]](images/1128.png)
![Br[Br-]Br](images/1129.png)
![[CH][NH2+]](images/1130.png)
![FF[O-]](images/1131.png)
![[CH]C=[CH+]](images/1132.png)
![ClO([O])[O-]](images/1133.png)
![[CH]C=[CH-]](images/1134.png)
![[CH][NH2-]](images/1135.png)
![[H][2H+]](images/1136.png)
![C[N-]](images/1137.png)
![ClCl[O+]](images/1138.png)
![C=[N+]](images/1139.png)
![FF[F+]](images/1140.png)
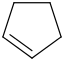

![C[Cl+]](images/1143.png)

![BrC#[N-]](images/1145.png)
![[Br]](images/1146.png)
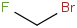
![ClCl[O-]](images/1148.png)
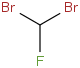
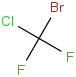
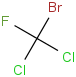
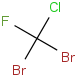
![[Kr+]](images/1153.png)
![ClCl([O])[O]](images/1154.png)
![[CH+]C](images/1155.png)
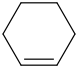
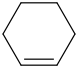
![F[C+]#CF](images/1158.png)
![C[CH-]C](images/1159.png)
![C(F)(F)=[O+]](images/1160.png)
![[C]=C=[C-]](images/1161.png)

![O[F+]](images/1163.png)
![Cl[C]OCl](images/1164.png)
![N[O-]](images/1165.png)
![Cl[C]OH](images/1166.png)
![H[C]OCl](images/1167.png)
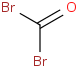

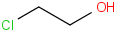
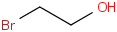

![c1cccc[c+]1](images/1173.png)

![c1[c]ccc[c-]1](images/1175.png)
![c1c[c]cc[c-]1](images/1176.png)
![[C-]1=C=C=1](images/1177.png)
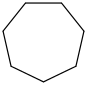
![[C+]N](images/1179.png)
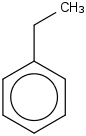
[O-]](images/1181.png)
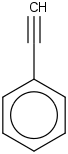
![O[Cl+]](images/1183.png)
![c1ccc(cc1)[O+]](images/1184.png)


![C=[CH2-]](images/1187.png)
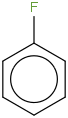
![Br[O+]](images/1189.png)
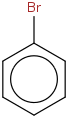

![C[CH-]O](images/1192.png)
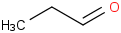




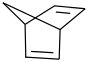
![OC(=O)CCC(=O)[O-].[NH4+]](images/1199.png)
![[O-]C(=O)CCC(=O)[O-].[NH4+].[NH4+]](images/1200.png)
![[NH-]=O](images/1201.png)
[O]](images/1202.png)
![[N-]O](images/1203.png)
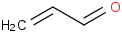

![O=C=C=[O+]](images/1206.png)

![[F+]](images/1208.png)
![O[Br+]](images/1209.png)
O[N+](=O)[O-]](images/1210.png)
![[CH]C=C=N](images/1211.png)
![[CH]C=C=N](images/1212.png)
![[CH]=CN=[CH]](images/1213.png)
![[CH]=CN=[CH]](images/1214.png)
![O[Cl-]](images/1215.png)
![C=[C+]C#N](images/1216.png)

![N[NH+]](images/1218.png)
![CO[CH2+]](images/1219.png)
![CO[CH2-]](images/1220.png)
![C1C[CH-]1](images/1221.png)
![CC=[O-]](images/1222.png)

![C=C=[C+]=C](images/1224.png)
![[C]=C1C#C1](images/1225.png)
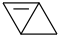
![C=CC=[C-]](images/1227.png)
![C12C3C1[CH+]23](images/1228.png)
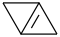
![C1[C+]2=C1C2](images/1230.png)

![[C-]=C1C#C1](images/1232.png)
![[NH-]](images/1233.png)
![C#CC=[CH]](images/1234.png)
![O[F-]](images/1235.png)
![OO[O+]](images/1236.png)
![C=[C]O](images/1237.png)
![C=CC#[C+]](images/1238.png)
![C=C1C=[C+]1](images/1239.png)
![[N]1N=[N+]1](images/1240.png)
![[CH+]=C1C=C1](images/1241.png)
![[C+]1=CC=C1](images/1242.png)
![N#CN=[N+]=[N-]](images/1243.png)

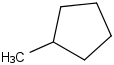
![[C]](images/1246.png)
![O=[Si]=O](images/1247.png)
![O=[Si]=O](images/1248.png)
![C=[O-]](images/1249.png)
![[CH]=C=[CH]](images/1250.png)
![[NH4+].[Cl-]](images/1251.png)
![[SiH2-]](images/1252.png)
![[SiH]](images/1253.png)
![[SiH+]](images/1254.png)
![[CH2+][N+]#[C-]](images/1255.png)
![[C]](images/1256.png)
![CC=[C]](images/1257.png)
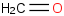

![C=C=[O+]](images/1260.png)
![C=C=[O-]](images/1261.png)

![FF[O+]](images/1263.png)
![[NH4]](images/1264.png)
![C#[CH-]](images/1266.png)
![O[N+]=O](images/1267.png)
![O=[O+]=O](images/1268.png)
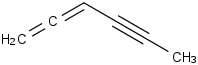
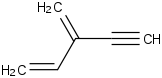
![[CH+]=C=O](images/1271.png)
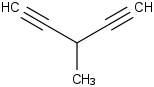
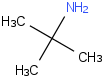
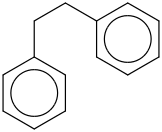
![[O]Br[O]](images/1277.png)
![OO[N]](images/1280.png)
![C12[C]=[C]2=[C]1](images/1281.png)
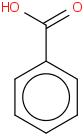
![N[NH2+]](images/1283.png)

=O](images/1285.png)
![[N]=[C+]=[N]](images/1286.png)
![[N]=[C-]=[N]](images/1287.png)
![[C+]1OO1](images/1288.png)



![[C+]=N=[N]](images/1292.png)
![[C-]=N=[N]](images/1293.png)
![[C]1N=N1](images/1294.png)
![[C-]1OO1](images/1295.png)
![CC#C[CH2+]](images/1296.png)
![[C+]Cl](images/1297.png)
![[C-]Cl](images/1298.png)

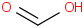
![OO[Cl-]](images/1301.png)

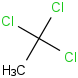
![[C-]#[O+]](images/1304.png)
![[N-]N](images/1305.png)
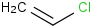
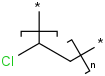
![[H]/N=[N-]\[H]](images/1308.png)
![[N+]1=NO1](images/1309.png)

![[C+]O[O]](images/1311.png)

![ClO[O-]](images/1313.png)

![C(=O)[O]](images/1315.png)
![F[H][F+]](images/1316.png)

![O1[CH]O1](images/1318.png)
![CC[CH3+]](images/1319.png)

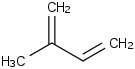
![[N]N=[N-]](images/1322.png)
F](images/1323.png)
![[H][H]](images/1324.png)
![[C-]F](images/1325.png)

![[C]=[C]](images/1327.png)
![[O+6]](images/1328.png)

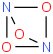

![BrBr[O]](images/1332.png)

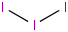
![IN=[C]](images/1335.png)
![[C]=C(F)F](images/1336.png)
![[C]=C=[C+]](images/1337.png)
![[C+]#CF](images/1338.png)


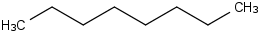
![N=[N]=[N-]](images/1342.png)

![Cl[C]OCl](images/1344.png)
![Cl[C+]=O](images/1345.png)
![[ClH-]](images/1346.png)
![[H][Cl](=O)(=O)=O](images/1347.png)
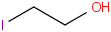
![C#CC#[CH+]](images/1349.png)
![ClO[Cl]=O](images/1350.png)
![O=C=[C+]=C=O](images/1351.png)
![O=C=[C-]=C=O](images/1352.png)
![c1ccc(cc1)[H-]](images/1353.png)


=O](images/1356.png)
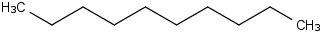
![C1=[C-]=C1](images/1358.png)
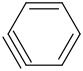
![OO[O]](images/1360.png)
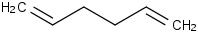

![c1[c]ccc[c]1](images/1363.png)
![C[C+]C](images/1364.png)
![c1c[c]cc[c+]1](images/1365.png)
![c1c[c]cc[c]1](images/1366.png)
![FO[O+]F](images/1367.png)
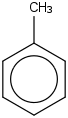
![FO[F-]](images/1369.png)
![[CH+]1C=CC=C1](images/1370.png)
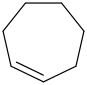
![[N]O[N+]](images/1372.png)
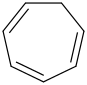
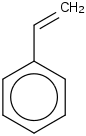

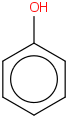
![C1=C[CH-]1](images/1377.png)
![[H][H+]](images/1378.png)

![[F+]F[H]](images/1380.png)
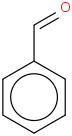
![C[C-]C](images/1382.png)
![[CH][CH][C+]](images/1383.png)
![c1ccc(cc1)[F-]](images/1384.png)
![c1(Cl)c[c+]ccc1](images/1385.png)
![c1(Cl)cc[c+]cc1](images/1386.png)
![c1(Cl)[c+]cccc1](images/1387.png)
![[CH2]C[CH2]](images/1388.png)
![O.[H]](images/1389.png)
![c1ccc(cc1)[I+]](images/1390.png)
[O-]](images/1391.png)

[O-]](images/1393.png)

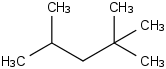
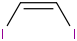

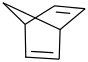


[O-].O.O.O](images/1401.png)


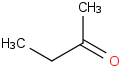
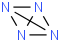

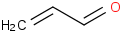
![C12=[C]C2=[C+]1](images/1408.png)
![C12=[C]C2=[C-]1](images/1409.png)
![O=C=C=[O-]](images/1410.png)
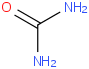
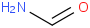
![O=[Cl+]=O](images/1413.png)
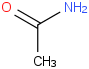
![O=NON=[O-]](images/1415.png)
[O-].O](images/1416.png)

![CC#[C+]](images/1418.png)
![CC=[CH+]](images/1419.png)
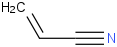
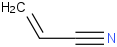
![[C]#CC#[C-]](images/1422.png)
![C=C[N+]#[C-]](images/1423.png)
![CC=[CH-]](images/1424.png)
![C[C][N+]#[C-]](images/1425.png)
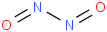

![N=[N+]=[N]](images/1428.png)
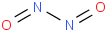
![[C+]=C](images/1430.png)
![C1C[CH+]1](images/1431.png)
![[N]O[N-]](images/1432.png)


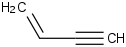
[O-]](images/1436.png)
[O]](images/1437.png)

![IN=[C-]](images/1439.png)
![C=CC=[C]](images/1440.png)
![[C+]O[O]](images/1441.png)
![FO[F+]](images/1442.png)
![C1C=[C+]1](images/1443.png)
![[C+]=C1C#C1](images/1444.png)
![C#CC=[CH+]](images/1445.png)
=O](images/1446.png)
![BrN=[C]](images/1447.png)
![[H]1[H][H]1](images/1448.png)
![ClO[O]](images/1449.png)
![[CH][CH][C-]](images/1450.png)
![[CH+]=C1C=C1](images/1451.png)
![O[N+]=O](images/1452.png)
![[C+]1=CC=C1](images/1453.png)
![[C-]1=CC=C1](images/1454.png)
![C1[CH+]C=C1](images/1455.png)
![[CH]=CO](images/1456.png)
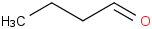
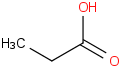
![[CH]=CO](images/1459.png)
![[CH]=CO](images/1460.png)
![[BrH+]](images/1461.png)
![ClN=[C]](images/1462.png)
![[CH+]C=O](images/1463.png)
![[H][2H]](images/1464.png)
![[Si][Si+]](images/1465.png)
![O=[Si+]=O](images/1466.png)
![O=[Si-]=O](images/1467.png)

![OOO[O]](images/1469.png)
![[Si-]=O](images/1470.png)

![[Si]=[C]](images/1472.png)
![[SiH4+]](images/1473.png)

![[SiH3+]](images/1475.png)
![[O][O](F)F](images/1476.png)
![[NH3-]](images/1478.png)
[O-]](images/1479.png)
![OO[Cl+]](images/1480.png)



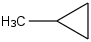
![O[N-]=O](images/1485.png)


![[C]#[O-]](images/1488.png)
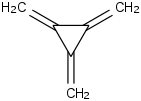
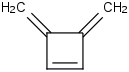

![[CH-]C=O](images/1492.png)
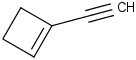
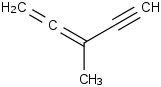


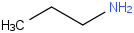

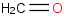
![N#CC#[N-]](images/1500.png)
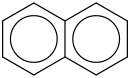
![[C]#[N+][N+]#[C-]](images/1502.png)
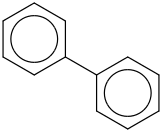
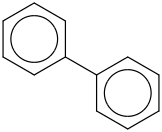
![FN=[C]](images/1505.png)
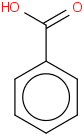
![[He][H+]](images/1507.png)
![[Xe+][H]](images/1508.png)
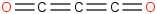
![C(=O)O[O-]](images/1510.png)
![Br[Br-]Br](images/1511.png)
![C(=O)O[O]](images/1512.png)
![[O+4]](images/1513.png)
![C[N+]](images/1514.png)
![N=[N+]=[N-]](images/1515.png)
![c1[c]ccc[c]1](images/1516.png)
![[CH]C=[CH]](images/1517.png)
![[O+7]](images/1518.png)

![C[CH]O](images/1520.png)
=O](images/1521.png)
![C(=O)[N]](images/1522.png)
![[CH-]1C=CC=CC=C1](images/1523.png)

![CC[CH+]C](images/1525.png)

[O-]](images/1527.png)
![Cl[O+]Cl](images/1528.png)
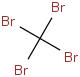

![N=[N]=[N-]](images/1531.png)
![Cl[O-]Cl](images/1532.png)
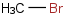
![O1O[O+]1](images/1534.png)


![FO[O+]F](images/1537.png)


![[F]](images/1540.png)
![[N]](images/1541.png)
![N=[N+]=[N-]](images/1542.png)
![[CH]O](images/1543.png)

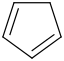


![[CH2]](images/1548.png)
![OO[Cl-]](images/1549.png)

![ClO([O])[O]](images/1551.png)
![[O]F[O-]](images/1552.png)
![[H]1[H+][H]1](images/1553.png)


![Br[Br+]](images/1556.png)
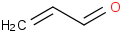
![[CH2+]=C=C=O](images/1558.png)
![C[C+]=C](images/1559.png)
![C#[C+]C=O](images/1560.png)
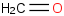
![[H][H-]](images/1562.png)
![O[OH+]](images/1563.png)
![C12=[C]C2=[C]1](images/1564.png)
![C[C-]=C](images/1565.png)
![[CH2+]CO](images/1566.png)
![[CH]=NO](images/1567.png)
![O[OH+]](images/1568.png)
![[NH4+].[OH-]](images/1569.png)
![C#CC#[N+]](images/1570.png)
![C#C[N+]#[C]](images/1571.png)
![[C]#CC#[C+]](images/1572.png)
![[C+]#CC#N](images/1573.png)

![[C]#CC#[C]](images/1575.png)
![[C]=[C]](images/1576.png)


![[CH]C=C=N](images/1579.png)
![CC=[CH+]](images/1580.png)

![[CH]=CN=[CH]](images/1582.png)
![CC=[CH-]](images/1583.png)

![[H]1[H][H]1](images/1585.png)
![[O++]](images/1586.png)
![CC#C[CH2+]](images/1587.png)
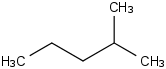
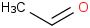




![C=C[C+]](images/1594.png)
![CC(=O)[O]](images/1595.png)
![O=NON=[O-]](images/1596.png)
![[2H-]](images/1597.png)
![CC(=O)[O]](images/1598.png)

![CC=C=[C+]](images/1600.png)
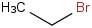
![C1[C-]2=C1C2](images/1602.png)

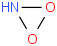
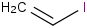
![C#[CH-]](images/1606.png)
![[C]=C=[C+]](images/1607.png)
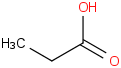
![[O+3]](images/1609.png)
![[C+]=C(F)F](images/1610.png)
![[H]1[H][H]1](images/1611.png)
![[2H][2H+]](images/1612.png)
![[Cl]](images/1613.png)
[O-]](images/1614.png)
![BrN=[C-]](images/1615.png)
![[H][O+][H]](images/1616.png)
![O=[Si]=O](images/1617.png)
![BrO[O]](images/1618.png)
![[Si+]=O](images/1619.png)
![CC=[CH2+]](images/1620.png)
![[OH3+]](images/1621.png)
![O[N+]=O](images/1622.png)
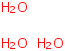
![O[N-]=O](images/1624.png)
![C1[CH2+]C1](images/1625.png)
![[SiH3][O+]](images/1626.png)
![ClO[O+]](images/1627.png)
![[SiH2+]O](images/1628.png)
![FF[O]](images/1629.png)
![F[F+]](images/1630.png)
![Cl[C]OCl](images/1631.png)
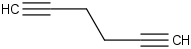
![[O+8]](images/1633.png)
![[I]](images/1634.png)
![[O]F[O+]](images/1635.png)

![[CH+]C=O](images/1637.png)
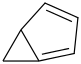
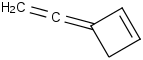
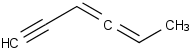
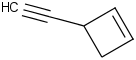

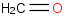
![[CH-]C=O](images/1644.png)

![OO[Cl+]](images/1646.png)
![[O+5]](images/1647.png)
![Br[Br+]Br](images/1648.png)
![[H][H+]](images/1649.png)
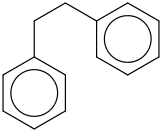
![[C]#[N+]C#N](images/1651.png)
F](images/1652.png)
![[C-]#[O+]](images/1653.png)
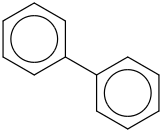


![[C]#[N+][N+]#[C-]](images/1657.png)
![[CH]1[C][C][C]1](images/1658.png)
![[H][H]](images/1659.png)
![[CH]C=[CH]](images/1660.png)
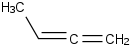
![O=[C+]1C=C1](images/1662.png)
![c1ccc(cc1)[OH+]](images/1663.png)


![[OH3+]](images/1666.png)
![c1ccc(cc1)[OH-]](images/1667.png)
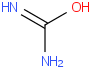
![O[C]=O](images/1669.png)
(=O)=O](images/1670.png)
![[NH2-]=C=O](images/1671.png)
O](images/1672.png)
![N=[C]O](images/1673.png)
![N=[C]O](images/1674.png)
![N=[C]O](images/1675.png)
![N=[C]O](images/1676.png)
![[CH2+]CO](images/1677.png)
![O.[OH2+]](images/1678.png)

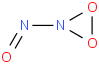
![[CH]=NO](images/1681.png)
![[CH]=NO](images/1682.png)
![O[OH+]](images/1683.png)
Cl](images/1684.png)
![OO[N]](images/1685.png)
![[C]=C(Cl)Cl](images/1686.png)
![OOO[O]](images/1687.png)
![[CH]O[O]](images/1688.png)

![[CH+]C=O](images/1690.png)
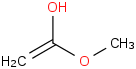
![[C]=CCl](images/1692.png)
![c1ccc(cc1)C=[O+]](images/1693.png)
![[Br]](images/1694.png)
![c1ccc(cc1)C=[O-]](images/1695.png)
![C12=[C]C2=[C]1](images/1696.png)
![O[OH+]](images/1697.png)
![[H]1[H][H]1](images/1698.png)
![Cl[C+]#N](images/1699.png)

![[CH]C=[CH]](images/1701.png)
![C#[C-]O](images/1702.png)

![C=[CH2-]](images/1704.png)
![[N]](images/1705.png)


![[C]=C1N=N1](images/1708.png)
![C[CH3-]](images/1709.png)
![C[C]C#N](images/1710.png)
![FO[O+]F](images/1711.png)
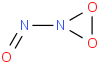
![[OH+]C#N](images/1713.png)


![N=[N+]=[N-]](images/1716.png)
![FO[O-]F](images/1717.png)
![[CH]C=C=N](images/1718.png)
![[CH-]#N](images/1719.png)
![[CH]C=C=N](images/1720.png)
![[CH]C=[CH]](images/1721.png)

![Cl[Cl+]](images/1723.png)
![C[F+]](images/1724.png)
![[CH]=CN=[CH]](images/1725.png)
![FF[O-]](images/1726.png)
![[CH]=CN=[CH]](images/1727.png)
![I[C+]#N](images/1728.png)
![[C]](images/1729.png)
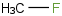
![N=[NH-]](images/1731.png)
![[N]=[N]=C=[C+]](images/1732.png)
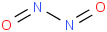
![[H]1[H][H]1](images/1734.png)
![[NH]](images/1735.png)
![[C]=C=O](images/1736.png)
![C[C]C](images/1737.png)
![N=[NH-]](images/1738.png)
![CO[C+]=O](images/1739.png)
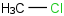
![[CH]=CC#N](images/1741.png)
![[CH+]=CC#N](images/1742.png)
![[CH-]=CC#N](images/1743.png)
![CO[C]=O](images/1744.png)
![O=NO[N+]=O](images/1745.png)
![[CH]=C[N+]#[C-]](images/1746.png)
![CC[CH-]](images/1747.png)
![FF[O+]](images/1748.png)
![[CH+]C#N](images/1749.png)
![[CH-]C#N](images/1750.png)
![[CH]C#N](images/1751.png)
![[C]#CC#[C]](images/1752.png)
![CO[C]=O](images/1753.png)
![[CH+][N+]#[C-]](images/1754.png)
![[CH-][N+]#[C-]](images/1755.png)
![[CH][N+]#[C-]](images/1756.png)
![C[Br+]](images/1757.png)
![[C+]=C=C=O](images/1758.png)
![[C-]=C=C=O](images/1759.png)
Cl](images/1760.png)
[O-]](images/1761.png)
![CO[C]=O](images/1762.png)
![[OH-]C#N](images/1763.png)
![[C-]=N](images/1764.png)
![N[NH-]](images/1765.png)
![CC[C]](images/1766.png)
Cl](images/1767.png)
![[H][H].[O]](images/1768.png)
![FF[O]](images/1769.png)
![[CH-]C=O](images/1770.png)
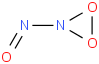
![C1=CC=[CH+]1](images/1772.png)
![CC(=O)[O+]](images/1773.png)
O](images/1774.png)
![CC1=C[C]1](images/1775.png)
![CC1=C[C]1](images/1776.png)

![C[I+]](images/1778.png)
![[OH2+]O](images/1779.png)
![C[N+]#[C-]](images/1780.png)

![N=[NH+]](images/1782.png)
![C=CC=[C]](images/1783.png)
![C=CC=[C]](images/1784.png)
![[CH]C](images/1785.png)
![C=CC=[C]](images/1786.png)
![C=CC=[C+]](images/1787.png)
![C=CC=[C+]](images/1788.png)
![C=CC=[C-]](images/1789.png)
![C=CC=[C-]](images/1790.png)
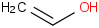

![CC=C=[C]](images/1793.png)
![CC=C=[C]](images/1794.png)

O](images/1796.png)
![[N-]](images/1797.png)
![O[OH+]](images/1798.png)
![[CH2]CO](images/1799.png)
![C=C[OH+]](images/1800.png)
![C(=O)O[O]](images/1801.png)
![N[NH2-]](images/1802.png)
![O=CO[CH2]](images/1803.png)
![C=C=C=[CH+]](images/1804.png)
[O]](images/1805.png)
![[CH2]C(=O)O](images/1806.png)
![C1[C]O1](images/1807.png)
![OOO[O]](images/1808.png)
![[BrH-]](images/1809.png)
![[CH2]C(=O)OC](images/1810.png)
![C#CC=[CH]](images/1811.png)
![C#CC=[CH+]](images/1812.png)
![C#CC=[CH-]](images/1813.png)

![[NH]](images/1815.png)

![C=CC#[C+]](images/1817.png)
(F)(F)F](images/1818.png)
![[Cl]](images/1819.png)
![CC(=O)O[CH2]](images/1820.png)

![C=C1C=[C+]1](images/1822.png)
![[NH]O](images/1823.png)
![ClN=[C-]](images/1824.png)
![CC#C[CH2+]](images/1825.png)
![[CH+]=C1C=C1](images/1826.png)
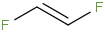
![[C]=C=[C-]](images/1828.png)
![[CH]Cl](images/1829.png)

![[C+]1=CC=C1](images/1831.png)
![[NH+]O](images/1832.png)
![[CH]=CO](images/1833.png)
![[C]=[O+][C]](images/1834.png)
![C1[CH-]C=C1](images/1835.png)
![ClN=[C+]](images/1836.png)

![[C-]=C(F)F](images/1838.png)
![[CH4-]](images/1839.png)
![c1ccc(cc1)O[O+]](images/1840.png)
![c1ccc(cc1)O[O-]](images/1841.png)

![[OH3+]](images/1843.png)
![[C]=C(Br)Br](images/1844.png)
![[C]=CF](images/1845.png)

![[CH2+]=C1C=CC=C1](images/1847.png)
![[CH2-]=C1C=CC=C1](images/1848.png)
![BrN=[C+]](images/1849.png)
![C[N+]](images/1850.png)


![[Si+]](images/1853.png)
![[Si-]](images/1854.png)

![[C]=CBr](images/1856.png)
![[Si][Si-]](images/1857.png)
O](images/1858.png)
![N=[NH+]](images/1859.png)
![C(F)(F)=[O-]](images/1860.png)

![O[O+]O](images/1862.png)
![FN=[C-]](images/1863.png)
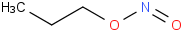
![I[I+]](images/1865.png)
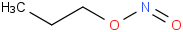
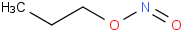
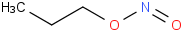
![[CH2+]C#N](images/1869.png)
![F[C]OF](images/1870.png)
![[Si][C-]](images/1871.png)
![N#N.[H-]](images/1872.png)
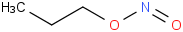

![[Si]=[C]](images/1875.png)
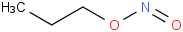
O](images/1877.png)
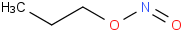
![I[O+]](images/1879.png)

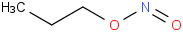
![OO[Cl+]](images/1882.png)
![C1[CH+]O1](images/1883.png)
![C(=O)[C+]=O](images/1884.png)
![[SiH2]](images/1885.png)
![C(=O)[C-]=O](images/1886.png)
![[C][O].[H-]](images/1887.png)
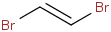
![C1[CH-]O1](images/1889.png)
![[SiH3][SiH3+]](images/1890.png)

![[CH]=C=[CH]](images/1892.png)
![O[C+]=C=O](images/1893.png)
![O[C-]=C=O](images/1894.png)
![C(=[O+])(Cl)Cl](images/1895.png)
![O[Cl+]=O](images/1896.png)

![[C]1CC1](images/1898.png)

F](images/1900.png)
![[H]1[H][H]1](images/1901.png)
![ClO[Cl]=O](images/1902.png)
![Cl[C]OCl](images/1903.png)

![[CH]C=O](images/1905.png)
![[SiH2-]O](images/1906.png)
![Cl[C]OCl](images/1907.png)
![CN[CH2-]](images/1908.png)
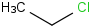
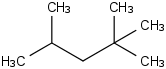
![C1=CC2C3C1[CH+]23](images/1911.png)
![F[C+]#N](images/1912.png)
![FC#[N-]](images/1913.png)


![[N]=[N]=C=[C-]](images/1916.png)
![[H][O-][H]](images/1917.png)
![C(=O)O[O-]](images/1918.png)
![OO[Cl-]](images/1919.png)
![[H][O+][H]](images/1920.png)
![Cl[C]OH](images/1921.png)
![Cl[C]OH](images/1922.png)
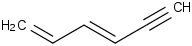
![[CH-]1C=CC=C1](images/1924.png)
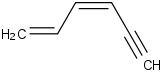
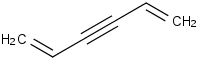
![C#[C+]O](images/1927.png)
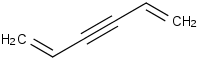
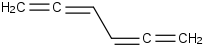
![O[N-]=O](images/1930.png)
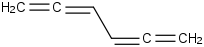
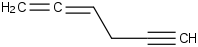
![[H]1[H+][H]1](images/1933.png)
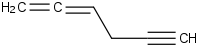
![N=[N]=[N-]](images/1935.png)
![H[C]OCl](images/1936.png)
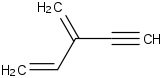
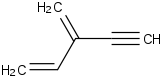
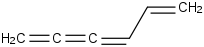
![FN=[C+]](images/1940.png)
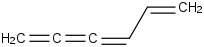
![H[C]OCl](images/1942.png)
![[OH+][N+]#[C-]](images/1943.png)
![CN(C)[CH2-]](images/1944.png)


![C(=O)[N]](images/1947.png)
![O=NON=[O-]](images/1948.png)
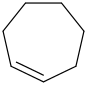
![[CH]C=[CH]](images/1950.png)
![[CH]C=C](images/1951.png)
![[NH-][O+]=O](images/1952.png)
[O]](images/1953.png)
![C/C=C\[CH2]](images/1954.png)
![CC=C[CH2]](images/1955.png)

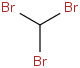
![Cl[C-]=O](images/1958.png)
![[CH]C=C](images/1959.png)
![[C-]N](images/1960.png)

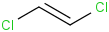
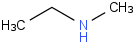

![[CH]=N](images/1965.png)
![[CH-]O](images/1966.png)
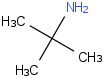


![[CH]C=C](images/1970.png)
![c1c(cc2ccccc2c1)[H+]](images/1971.png)
![c1c(cc2ccccc2c1)[H-]](images/1972.png)
![[NH-]=C=O](images/1973.png)
![[C]=O.[C]](images/1974.png)
![N#[N-]](images/1975.png)
![[C-]#[N]C#N](images/1976.png)
![ClC#[N-]](images/1977.png)
![[CH-]=N](images/1978.png)
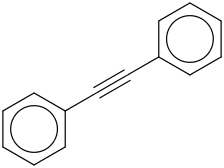
[O-]](images/1980.png)

![[O--]](images/1982.png)

![Br[C+]#N](images/1984.png)
![[H][H].[O]](images/1985.png)

![[CH+]O](images/1987.png)
![[C+]#CC#C](images/1988.png)
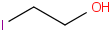

![[C-]#[N+][N]#[C-]](images/1991.png)
![[C]#[N+][N+]#[C-]](images/1992.png)


![[F]](images/1995.png)
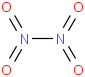

![O[Cl-]=O](images/1998.png)
![[OH2+]O](images/1999.png)
(F)(F)F](images/2000.png)
![C[N+]](images/2001.png)
![C=C=C=[O-]](images/2002.png)
[O-]](images/2003.png)
![[Kr++]](images/2004.png)
![C#C[CH-]](images/2005.png)
![[Xe++]](images/2006.png)
![[C]](images/2007.png)
![[Ne++]](images/2008.png)
![[Ne+3]](images/2009.png)
![[Ne+4]](images/2010.png)
![[Ne+5]](images/2011.png)
![[Ne+6]](images/2012.png)
![[Ne+7]](images/2013.png)
![[Ne+8]](images/2014.png)
![[Ne+9]](images/2015.png)
![[Ne+]](images/2016.png)
![[Ne+10]](images/2017.png)
![[Ar][Ar+]](images/2018.png)
![[Ar+]](images/2019.png)
![[Ar][Ar]](images/2020.png)
![[He+]](images/2021.png)
![[He++]](images/2022.png)
![[He-]](images/2023.png)
![[He-]](images/2024.png)
![[Ar-]](images/2025.png)
![[Ar-]](images/2026.png)
![[He-]](images/2027.png)
![[He-]](images/2028.png)
![[He-]](images/2029.png)
![[Ar-]](images/2030.png)
![[H+]](images/2031.png)