Selected ATcT [1, 2] enthalpy of formation based on version 1.176 of the Thermochemical Network [3]This version of ATcT results[3] was generated by additional expansion of version 1.172 to include species related to Criegee intermediates that are involved in several ongoing studies[4].
|
Benzyl |
| Formula: C6H5CH2 (g) |
| CAS RN: 2154-56-5 |
| ATcT ID: 2154-56-5*0 |
| SMILES: c1ccc(cc1)[CH2] |
| InChI: InChI=1S/C7H7/c1-7-5-3-2-4-6-7/h2-6H,1H2 |
| InChIKey: SLRMQYXOBQWXCR-UHFFFAOYSA-N |
| Hills Formula: C7H7 |
2D Image: |
![c1ccc(cc1)[CH2]](../images/159.png) |
| Aliases: C6H5CH2; Benzyl; Phenylmethyl; Benzyl radical |
| Relative Molecular Mass: 91.1305 ± 0.0056 |
| ΔfH°(0 K) | ΔfH°(298.15 K) | Uncertainty | Units |
|---|
| 230.13 | 211.32 | ± 0.51 | kJ/mol |
|
3D Image of C6H5CH2 (g) |
| |
| spin ON spin OFF |
| |
Top contributors to the provenance of ΔfH° of C6H5CH2 (g)The 20 contributors listed below account only for 55.6% of the provenance of ΔfH° of C6H5CH2 (g).
A total of 255 contributors would be needed to account for 90% of the provenance.
Please note: The list is limited to 20 most important contributors or, if less, a number sufficient to account for 90% of the provenance. The Reference acts as a further link to the relevant references and notes for the measurement. The Measured Quantity is normaly given in the original units; in cases where we have reinterpreted the original measurement, the listed value may differ from that given by the authors. The quoted uncertainty is the a priori uncertainty used as input when constructing the initial Thermochemical Network, and corresponds either to the value proposed by the original authors or to our estimate; if an additional multiplier is given in parentheses immediately after the prior uncertainty, it corresponds to the factor by which the prior uncertainty needed to be multiplied during the ATcT analysis in order to make that particular measurement consistent with the prevailing knowledge contained in the Thermochemical Network.
|
Contribution
(%) | TN
ID | Reaction | Measured Quantity | Reference | | 24.7 | 6960.1 | [C6H5CH2]- (g) → C6H5CH2 (g) | ΔrH°(0 K) = 0.912 ± 0.006 eV | Gunion 1992 | | 5.5 | 6956.1 | C6H5CH3 (l) + 9 O2 (g) → 7 CO2 (g) + 4 H2O (cr,l) | ΔrH°(298.15 K) = -934.49 ± 0.12 kcal/mol | Prosen 1945a, as quoted by Cox 1970 | | 5.5 | 6956.4 | C6H5CH3 (l) + 9 O2 (g) → 7 CO2 (g) + 4 H2O (cr,l) | ΔrH°(298.15 K) = -934.45 ± 0.12 kcal/mol | Good 1969 | | 5.0 | 9186.1 | C6H5CH2CH2C6H5 (g) → 2 C6H5CH2 (g) | ΔrG°(1200 K) = 82.5 ± 4 kJ/mol | Hippler 1990, Muller-Markgraf 1988, 3rd Law, est unc | | 1.8 | 9186.3 | C6H5CH2CH2C6H5 (g) → 2 C6H5CH2 (g) | ΔrH°(0 K) = 67.13 ± 1.60 kcal/mol | Ruscic G4 | | 1.1 | 2228.7 | C (graphite) + O2 (g) → CO2 (g) | ΔrH°(298.15 K) = -393.464 ± 0.024 kJ/mol | Hawtin 1966, note CO2e | | 0.9 | 9186.4 | C6H5CH2CH2C6H5 (g) → 2 C6H5CH2 (g) | ΔrH°(0 K) = 68.25 ± 2.16 kcal/mol | Ruscic CBS-n | | 0.9 | 6966.5 | C6H5CH2 (g) + CH3CH3 (g) → C6H5CH3 (g) + CH3CH2 (g) | ΔrH°(0 K) = 10.34 ± 0.85 kcal/mol | Ruscic W1RO | | 0.9 | 6971.5 | [C6H5CH2]+ (g) + CH3CH3 (g) → C6H5CH3 (g) + [CH3CH2]+ (g) | ΔrH°(0 K) = 30.17 ± 0.85 kcal/mol | Ruscic W1RO | | 0.8 | 6965.5 | C6H5CH3 (g) + CH3 (g) → C6H5CH2 (g) + CH4 (g) | ΔrH°(0 K) = -14.13 ± 0.9 kcal/mol | Ruscic W1RO | | 0.8 | 6966.4 | C6H5CH2 (g) + CH3CH3 (g) → C6H5CH3 (g) + CH3CH2 (g) | ΔrH°(0 K) = 9.99 ± 0.90 kcal/mol | Ruscic CBS-n | | 0.8 | 6966.2 | C6H5CH2 (g) + CH3CH3 (g) → C6H5CH3 (g) + CH3CH2 (g) | ΔrH°(0 K) = 10.23 ± 0.90 kcal/mol | Ruscic G4 | | 0.8 | 6970.7 | C6H5CH3 (g) → [C6H5CH2]+ (g) + H (g) | ΔrH°(0 K) = 11.134 ± 0.040 eV | Ruscic W1RO | | 0.8 | 6971.1 | [C6H5CH2]+ (g) + CH3CH3 (g) → C6H5CH3 (g) + [CH3CH2]+ (g) | ΔrH°(0 K) = 30.77 ± 0.90 kcal/mol | Ruscic G3X | | 0.8 | 6971.2 | [C6H5CH2]+ (g) + CH3CH3 (g) → C6H5CH3 (g) + [CH3CH2]+ (g) | ΔrH°(0 K) = 30.09 ± 0.90 kcal/mol | Ruscic G4 | | 0.8 | 6971.4 | [C6H5CH2]+ (g) + CH3CH3 (g) → C6H5CH3 (g) + [CH3CH2]+ (g) | ΔrH°(0 K) = 29.78 ± 0.90 kcal/mol | Ruscic CBS-n | | 0.8 | 6956.2 | C6H5CH3 (l) + 9 O2 (g) → 7 CO2 (g) + 4 H2O (cr,l) | ΔrH°(298.15 K) = -934.72 ± 0.11 (×2.828) kcal/mol | Coops 1946, as quoted by Cox 1970 | | 0.7 | 6963.1 | C6H5CH3 (g) → C6H5CH2 (g) + H (g) | ΔrG°(1000 K) = 252.4 ± 4 kJ/mol | Hippler 1990, 3rd Law, note unc | | 0.7 | 6965.2 | C6H5CH3 (g) + CH3 (g) → C6H5CH2 (g) + CH4 (g) | ΔrH°(0 K) = -14.19 ± 1.0 kcal/mol | Ruscic G4 | | 0.7 | 6965.4 | C6H5CH3 (g) + CH3 (g) → C6H5CH2 (g) + CH4 (g) | ΔrH°(0 K) = -13.68 ± 1.0 kcal/mol | Ruscic CBS-n |
|
Top 10 species with enthalpies of formation correlated to the ΔfH° of C6H5CH2 (g) |
Please note: The correlation coefficients are obtained by renormalizing the off-diagonal elements of the covariance matrix by the corresponding variances.
The correlation coefficient is a number from -1 to 1, with 1 representing perfectly correlated species, -1 representing perfectly anti-correlated species, and 0 representing perfectly uncorrelated species.
|
Correlation
Coefficent
(%) | Species Name | Formula | Image | ΔfH°(0 K) | ΔfH°(298.15 K) | Uncertainty | Units | Relative
Molecular
Mass | ATcT ID | | 99.3 | Benzylium | [C6H5CH2]+ (g) | ![c1ccc(cc1)[CH2+]](../images/935.png) | 929.56 | 910.06 | ± 0.51 | kJ/mol | 91.1299 ±
0.0056 | 6711-19-9*0 | | 55.8 | Benzylide | [C6H5CH2]- (g) | ![c1ccc(cc1)[CH2-]](../images/607.png) | 141.98 | 123.07 | ± 0.39 | kJ/mol | 91.1310 ±
0.0056 | 18860-15-6*0 | | 50.0 | Toluene | C6H5CH3 (g) | 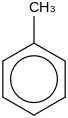 | 73.35 | 50.06 | ± 0.31 | kJ/mol | 92.1384 ±
0.0056 | 108-88-3*0 | | 49.1 | Toluene | C6H5CH3 (l) | 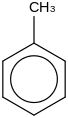 | 19.78 | 12.03 | ± 0.31 | kJ/mol | 92.1384 ±
0.0056 | 108-88-3*500 | | 28.5 | Benzylidene | C6H5CH (g) | ![c1ccc(cc1)[CH]](../images/2297.png) | 473.0 | 457.7 | ± 1.3 | kJ/mol | 90.1225 ±
0.0056 | 3101-08-4*0 | | 28.5 | Benzylidene | C6H5CH (g, triplet) | ![c1ccc(cc1)[CH]](../images/936.png) | 473.0 | 457.7 | ± 1.3 | kJ/mol | 90.1225 ±
0.0056 | 3101-08-4*1 | | 25.6 | 4,5-Bis(methylene)-2-cyclopenten-1-yl | CH(CHC(CH2)C(CH2)CH) (g) | ![[CH]1C(=C)C(=C)C=C1](../images/2059.png) | 315.3 | 297.1 | ± 2.0 | kJ/mol | 91.1305 ±
0.0056 | 302792-28-5*0 | | 25.5 | 2,5-Bis(methylene)-3-cyclopenten-1-yl | CH(C(CH2)CHCHC(CH2)) (g) | ![[CH]1C(=C)C=CC1(=C)](../images/2060.png) | 321.0 | 302.8 | ± 2.0 | kJ/mol | 91.1305 ±
0.0056 | 512782-58-0*0 | | 25.5 | 2-Methylphenyl | C6H4CH3 (g, ortho) | ![c1c[c]c(cc1)C](../images/1114.png) | 324.5 | 306.5 | ± 1.2 | kJ/mol | 91.1305 ±
0.0056 | 22904-44-5*0 | | 25.0 | 4-Methylphenyl | C6H4CH3 (g, para) | ![[c]1ccc(cc1)C](../images/1115.png) | 327.2 | 309.2 | ± 1.2 | kJ/mol | 91.1305 ±
0.0056 | 2396-02-3*0 |
|
Most Influential reactions involving C6H5CH2 (g)Please note: The list, which is based on a hat (projection) matrix analysis, is limited to no more than 20 largest influences.
|
Influence
Coefficient | TN
ID | Reaction | Measured Quantity | Reference | | 0.872 | 6959.2 | C6H5CH2 (g) → [C6H5CH2]+ (g) | ΔrH°(0 K) = 58468 ± 5 cm-1 | Eiden 1996 | | 0.560 | 6960.1 | [C6H5CH2]- (g) → C6H5CH2 (g) | ΔrH°(0 K) = 0.912 ± 0.006 eV | Gunion 1992 | | 0.144 | 7008.5 | CH(CHC(CH2)C(CH2)CH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -21.28 ± 1.2 kcal/mol | Ruscic W1RO | | 0.133 | 7010.5 | CH(C(CH2)CHCHC(CH2)) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -22.97 ± 1.2 (×1.044) kcal/mol | Ruscic W1RO | | 0.123 | 7010.2 | CH(C(CH2)CHCHC(CH2)) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -21.37 ± 1.3 kcal/mol | Ruscic G4 | | 0.123 | 7010.4 | CH(C(CH2)CHCHC(CH2)) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -20.43 ± 1.3 kcal/mol | Ruscic CBS-n | | 0.122 | 7008.4 | CH(CHC(CH2)C(CH2)CH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -20.28 ± 1.3 kcal/mol | Ruscic CBS-n | | 0.122 | 7008.2 | CH(CHC(CH2)C(CH2)CH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -20.01 ± 1.3 kcal/mol | Ruscic G4 | | 0.111 | 6959.1 | C6H5CH2 (g) → [C6H5CH2]+ (g) | ΔrH°(0 K) = 58456 ± 14 cm-1 | Eiden 1991, Eiden 1991a | | 0.111 | 6980.5 | C6H5CH (g, triplet) + CH3 (g) → C6H5CH2 (g) + CH2 (g, triplet) | ΔrH°(0 K) = -0.21 ± 0.85 kcal/mol | Ruscic W1RO | | 0.107 | 7028.5 | (CHCH)(CHCHCH)(CHCH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -60.39 ± 1.2 kcal/mol | Ruscic W1RO | | 0.106 | 7010.1 | CH(C(CH2)CHCHC(CH2)) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -21.31 ± 1.4 kcal/mol | Ruscic G3X | | 0.105 | 7008.1 | CH(CHC(CH2)C(CH2)CH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -19.89 ± 1.4 kcal/mol | Ruscic G3X | | 0.103 | 3993.5 | CH2CHC(CHCHCHCH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -21.41 ± 1.2 kcal/mol | Ruscic W1RO | | 0.099 | 6980.4 | C6H5CH (g, triplet) + CH3 (g) → C6H5CH2 (g) + CH2 (g, triplet) | ΔrH°(0 K) = -0.12 ± 0.90 kcal/mol | Ruscic CBS-n | | 0.099 | 6980.1 | C6H5CH (g, triplet) + CH3 (g) → C6H5CH2 (g) + CH2 (g, triplet) | ΔrH°(0 K) = -0.64 ± 0.90 kcal/mol | Ruscic G3X | | 0.099 | 6980.2 | C6H5CH (g, triplet) + CH3 (g) → C6H5CH2 (g) + CH2 (g, triplet) | ΔrH°(0 K) = -0.49 ± 0.90 kcal/mol | Ruscic G4 | | 0.093 | 7010.6 | CH(C(CH2)CHCHC(CH2)) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -22.6 ± 1.5 kcal/mol | Marti 2023, est unc | | 0.092 | 7008.6 | CH(CHC(CH2)C(CH2)CH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -20.9 ± 1.5 kcal/mol | Marti 2023, est unc | | 0.091 | 7028.4 | (CHCH)(CHCHCH)(CHCH) (g) → C6H5CH2 (g) | ΔrH°(0 K) = -61.00 ± 1.3 kcal/mol | Ruscic CBS-n |
|
|
|
References
|
|
1
|
|
B. Ruscic, R. E. Pinzon, M. L. Morton, G. von Laszewski, S. Bittner, S. G. Nijsure, K. A. Amin, M. Minkoff, and A. F. Wagner,
Introduction to Active Thermochemical Tables: Several "Key" Enthalpies of Formation Revisited.
J. Phys. Chem. A 108, 9979-9997 (2004)
[DOI: 10.1021/jp047912y]
|
|
2
|
|
B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner,
Active Thermochemical Tables: Thermochemistry for the 21st Century.
J. Phys. Conf. Ser. 16, 561-570 (2005)
[DOI: 10.1088/1742-6596/16/1/078]
|
|
3
|
|
B. Ruscic and D. H. Bross,
Active Thermochemical Tables (ATcT) values based on ver. 1.176 of the Thermochemical Network (2024); available at ATcT.anl.gov |
|
4
|
|
T. L. Nguyen et al, ongoing studies (2024)
|
|
5
|
|
B. Ruscic,
Uncertainty Quantification in Thermochemistry, Benchmarking Electronic Structure Computations, and Active Thermochemical Tables.
Int. J. Quantum Chem. 114, 1097-1101 (2014)
[DOI: 10.1002/qua.24605]
|
|
6
|
|
B. Ruscic and D. H. Bross,
Thermochemistry
Computer Aided Chem. Eng. 45, 3-114 (2019)
[DOI: 10.1016/B978-0-444-64087-1.00001-2]
|
|
|
|
Formula
|
|
The aggregate state is given in parentheses following the formula, such as: g - gas-phase, cr - crystal, l - liquid, etc.
|
|
|
Uncertainties
|
The listed uncertainties correspond to estimated 95% confidence limits, as customary in thermochemistry (see, for example, Ruscic [5] and Ruscic and Bross[6]).
Note that an uncertainty of ± 0.000 kJ/mol indicates that the estimated uncertainty is < ± 0.0005 kJ/mol.
|
|
|
Website Functionality Credits
|
The reorganization of the website was developed and implemented by David H. Bross (ANL).
The find function is based on the complete Species Dictionary entries for the appropriate version of the ATcT TN.
The molecule images are rendered by Indigo-depict.
The XYZ renderings are based on Jmol: an open-source Java viewer for chemical structures in 3D. http://www.jmol.org/.
|
|
|
Acknowledgement
|
|
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. DE-AC02-06CH11357.
|
![c1ccc(cc1)[CH2]](../images/159.png)
![c1ccc(cc1)[CH2+]](../images/935.png)
![c1ccc(cc1)[CH2-]](../images/607.png)


![c1ccc(cc1)[CH]](../images/2297.png)
![c1ccc(cc1)[CH]](../images/936.png)
![[CH]1C(=C)C(=C)C=C1](../images/2059.png)
![[CH]1C(=C)C=CC1(=C)](../images/2060.png)
![c1c[c]c(cc1)C](../images/1114.png)
![[c]1ccc(cc1)C](../images/1115.png)