Selected ATcT [1, 2] enthalpy of formation based on version 1.148 of the Thermochemical Network [3]This version of ATcT results[3] was generated by additional expansion of version 1.140 to include species relevant to a recent study of the role of atmospheric methanediol[4].
|
Ethyl |
| Formula: CH3CH2 (g) |
| CAS RN: 2025-56-1 |
| ATcT ID: 2025-56-1*0 |
| SMILES: C[CH2] |
| InChI: InChI=1S/C2H5/c1-2/h1H2,2H3 |
| InChIKey: QUPDWYMUPZLYJZ-UHFFFAOYSA-N |
| Hills Formula: C2H5 |
2D Image: |
![C[CH2]](../images/45.png) |
| Aliases: CH3CH2; Ethyl; Ethyl radical |
| Relative Molecular Mass: 29.0611 ± 0.0016 |
| ΔfH°(0 K) | ΔfH°(298.15 K) | Uncertainty | Units |
|---|
| 131.51 | 120.76 | ± 0.20 | kJ/mol |
|
3D Image of CH3CH2 (g) |
| |
| spin ON spin OFF |
| |
Top contributors to the provenance of ΔfH° of CH3CH2 (g)The 20 contributors listed below account only for 44.4% of the provenance of ΔfH° of CH3CH2 (g).
A total of 548 contributors would be needed to account for 90% of the provenance.
Please note: The list is limited to 20 most important contributors or, if less, a number sufficient to account for 90% of the provenance. The Reference acts as a further link to the relevant references and notes for the measurement. The Measured Quantity is normaly given in the original units; in cases where we have reinterpreted the original measurement, the listed value may differ from that given by the authors. The quoted uncertainty is the a priori uncertainty used as input when constructing the initial Thermochemical Network, and corresponds either to the value proposed by the original authors or to our estimate; if an additional multiplier is given in parentheses immediately after the prior uncertainty, it corresponds to the factor by which the prior uncertainty needed to be multiplied during the ATcT analysis in order to make that particular measurement consistent with the prevailing knowledge contained in the Thermochemical Network.
|
Contribution
(%) | TN
ID | Reaction | Measured Quantity | Reference | | 13.4 | 2412.3 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrG°(775 K) = 19.71 ± 0.10 kcal/mol | Brouard 1986, 3rd Law | | 6.5 | 2412.1 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrH°(675 K) = 153.82 ± 0.60 kJ/mol | Blitz 2021, Lightfoot 1987, Hanning-Lee 1993, Brouard 1986, Kurylo 1970, Michael 1973, Barker 1969, Barker 1970, Feng 1993, 2nd Law | | 3.7 | 2412.4 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrG°(800 K) = 19.17 ± 0.19 kcal/mol | Brouard 1986, 3rd Law | | 2.4 | 2412.5 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrG°(825 K) = 18.26 ± 0.11 (×2.134) kcal/mol | Brouard 1986, 3rd Law | | 2.2 | 2402.1 | CH3CH2 (g) → [CH3CH2]+ (g) | ΔrH°(0 K) = 8.117 ± 0.008 eV | Ruscic 1989b | | 2.0 | 2290.1 | 2 H2 (g) + C (graphite) → CH4 (g) | ΔrG°(1165 K) = 37.521 ± 0.068 kJ/mol | Smith 1946, note COf, 3rd Law | | 1.4 | 2402.15 | CH3CH2 (g) → [CH3CH2]+ (g) | ΔrH°(0 K) = 8.124 ± 0.010 eV | Lau 2005 | | 1.3 | 2406.1 | 2 CH4 (g) → CH3CH2 (g) + 3/2 H2 (g) | ΔrH°(0 K) = 264.07 ± 1.5 kJ/mol | Klippenstein 2017 | | 1.1 | 2443.1 | CH2CH2 (g) + 3 O2 (g) → 2 CO2 (g) + 2 H2O (cr,l) | ΔrH°(298.15 K) = -1411.18 ± 0.30 kJ/mol | Rossini 1937 | | 1.1 | 2597.6 | CH2NH2 (g) + CH4 (g) → CH3CH2 (g) + NH3 (g) | ΔrH°(0 K) = -0.52 ± 1.5 kJ/mol | Klippenstein 2017 | | 1.0 | 2415.6 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrH°(298.15 K) = -14.08 ± 0.28 (×1.189) kcal/mol | Dobis 1997, Seakins 1992, Nicovich 1991, Fettis 1960, 2nd Law | | 1.0 | 2416.3 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrG°(298.15 K) = -10.20 ± 0.15 (×2.229) kcal/mol | Dobis 1997, Fettis 1960, 3rd Law | | 1.0 | 125.2 | 1/2 O2 (g) + H2 (g) → H2O (cr,l) | ΔrH°(298.15 K) = -285.8261 ± 0.040 kJ/mol | Rossini 1939, Rossini 1931, Rossini 1931b, note H2Oa, Rossini 1930 | | 1.0 | 2408.10 | CH3CH2 (g) + CH4 (g) → CH3 (g) + CH3CH3 (g) | ΔrH°(0 K) = 16.37 ± 1.5 kJ/mol | Klippenstein 2017 | | 0.9 | 2414.2 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrH°(450 K) = -57.5 ± 1.5 kJ/mol | Ferrell 1998, 2nd Law, est unc | | 0.9 | 2414.1 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrG°(450 K) = -38.3 ± 1.5 kJ/mol | Ferrell 1998, 3rd Law, est unc | | 0.8 | 2383.1 | CH3CH3 (g) + 7/2 O2 (g) → 2 CO2 (g) + 3 H2O (cr,l) | ΔrH°(298.15 K) = -1560.68 ± 0.25 (×1.297) kJ/mol | Pittam 1972 | | 0.7 | 2464.10 | CH3CH (g, triplet) + CH3 (g) → CH2 (g, triplet) + CH3CH2 (g) | ΔrH°(0 K) = 11.49 ± 1.5 kJ/mol | Klippenstein 2017 | | 0.7 | 2414.4 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrH°(367 K) = -57.5 ± 1.7 kJ/mol | Seakins 1992, 2nd Law | | 0.6 | 2416.5 | CH3CH2 (g) + HBr (g) → CH3CH3 (g) + Br (g) | ΔrG°(298.15 K) = -10.12 ± 0.13 (×3.221) kcal/mol | Dobis 1997, Amphlett 1968, 3rd Law |
|
Top 10 species with enthalpies of formation correlated to the ΔfH° of CH3CH2 (g) |
Please note: The correlation coefficients are obtained by renormalizing the off-diagonal elements of the covariance matrix by the corresponding variances.
The correlation coefficient is a number from -1 to 1, with 1 representing perfectly correlated species, -1 representing perfectly anti-correlated species, and 0 representing perfectly uncorrelated species.
|
Correlation
Coefficent
(%) | Species Name | Formula | Image | ΔfH°(0 K) | ΔfH°(298.15 K) | Uncertainty | Units | Relative
Molecular
Mass | ATcT ID | | 41.0 | Ethylene | CH2CH2 (g) | 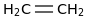 | 60.90 | 52.39 | ± 0.11 | kJ/mol | 28.0532 ±
0.0016 | 74-85-1*0 | | 41.0 | Ethylene cation | [CH2CH2]+ (g) | ![C=[CH2+]](../images/1101.png) | 1075.22 | 1068.01 | ± 0.11 | kJ/mol | 28.0526 ±
0.0016 | 34470-02-5*0 | | 39.4 | Ethane | CH3CH3 (g) |  | -68.38 | -84.00 | ± 0.12 | kJ/mol | 30.0690 ±
0.0017 | 74-84-0*0 | | 30.0 | Propane | CH3CH2CH3 (g) | 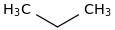 | -82.68 | -104.96 | ± 0.15 | kJ/mol | 44.0956 ±
0.0025 | 74-98-6*0 | | 28.1 | Ethylium | [CH3CH2]+ (g) | ![[CH2+]1[CH2][H]1](../images/106.png) | 915.18 | 903.11 | ± 0.29 | kJ/mol | 29.0606 ±
0.0016 | 14936-94-8*0 | | 23.0 | Propene | CH3CHCH2 (g) | 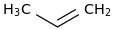 | 34.90 | 20.07 | ± 0.18 | kJ/mol | 42.0797 ±
0.0024 | 115-07-1*0 | | 23.0 | Propylene cation | [CH3CHCH2]+ (g) | ![CC=[CH2+]](../images/1545.png) | 975.19 | 961.66 | ± 0.18 | kJ/mol | 42.0792 ±
0.0024 | 34504-10-4*0 | | 22.8 | Carbon | C (g) | ![[C]](../images/20.png) | 711.404 | 716.889 | ± 0.040 | kJ/mol | 12.01070 ±
0.00080 | 7440-44-0*0 | | 22.8 | Carbon | C (g, triplet) | ![[C]](../images/1357.png) | 711.404 | 716.889 | ± 0.040 | kJ/mol | 12.01070 ±
0.00080 | 7440-44-0*1 | | 22.8 | Carbon | C (g, quintuplet) | ![[C]](../images/2614.png) | 1114.967 | 1120.113 | ± 0.040 | kJ/mol | 12.01070 ±
0.00080 | 7440-44-0*3 |
|
Most Influential reactions involving CH3CH2 (g)Please note: The list, which is based on a hat (projection) matrix analysis, is limited to no more than 20 largest influences.
|
Influence
Coefficient | TN
ID | Reaction | Measured Quantity | Reference | | 0.347 | 4556.6 | CH2CHOO (g, anti) + CH3CH2 (g) → CH3CH2OO (g, gauche) + CH2CH (g) | ΔrH°(0 K) = 44.97 ± 2.00 kJ/mol | Klippenstein 2017 | | 0.239 | 3600.5 | CH3CH2CH2CH2 (g) + [CH3CH2]+ (g) → [CH3CH2CH2CH2]+ (g) + CH3CH2 (g) | ΔrH°(0 K) = -0.614 ± 0.020 eV | Ruscic W1RO | | 0.237 | 5111.6 | CH2C(O)OH (g, syn) + CH3CH3 (g) → CH3C(O)OH (g, syn) + CH3CH2 (g) | ΔrH°(0 K) = 7.41 ± 2.00 kJ/mol | Klippenstein 2017 | | 0.236 | 7228.6 | CH2N(O)O (g) + CH3CH3 (g) → CH3N(O)O (g) + CH3CH2 (g) | ΔrH°(0 K) = -1.76 ± 2.0 kJ/mol | Klippenstein 2017 | | 0.217 | 5096.9 | HC(O)OCH2 (g, syn) + CH3CH3 (g) → HC(O)OCH3 (g, syn) + CH3CH2 (g) | ΔrH°(0 K) = 4.04 ± 2.00 kJ/mol | Klippenstein 2017 | | 0.186 | 2412.3 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrG°(775 K) = 19.71 ± 0.10 kcal/mol | Brouard 1986, 3rd Law | | 0.151 | 2402.1 | CH3CH2 (g) → [CH3CH2]+ (g) | ΔrH°(0 K) = 8.117 ± 0.008 eV | Ruscic 1989b | | 0.121 | 4504.6 | CH3CH2OO (g, trans) → CH3CH2 (g) + O2 (g) | ΔrH°(0 K) = 32.99 ± 0.60 kcal/mol | Wilke 2008, est unc | | 0.109 | 4556.5 | CH2CHOO (g, anti) + CH3CH2 (g) → CH3CH2OO (g, gauche) + CH2CH (g) | ΔrH°(0 K) = 10.86 ± 0.85 kcal/mol | Ruscic W1RO | | 0.106 | 2464.10 | CH3CH (g, triplet) + CH3 (g) → CH2 (g, triplet) + CH3CH2 (g) | ΔrH°(0 K) = 11.49 ± 1.5 kJ/mol | Klippenstein 2017 | | 0.105 | 4507.6 | CH3CH2OO (g, gauche) + CH3 (g) → CH3OO (g) + CH3CH2 (g) | ΔrH°(0 K) = 10.09 ± 2.00 kJ/mol | Klippenstein 2017 | | 0.099 | 4503.9 | CH3CH2OO (g, gauche) → CH3CH2 (g) + O2 (g) | ΔrH°(0 K) = 136.93 ± 2.00 kJ/mol | Klippenstein 2017 | | 0.098 | 4556.4 | CH2CHOO (g, anti) + CH3CH2 (g) → CH3CH2OO (g, gauche) + CH2CH (g) | ΔrH°(0 K) = 11.19 ± 0.90 kcal/mol | Ruscic CBS-n | | 0.098 | 4556.1 | CH2CHOO (g, anti) + CH3CH2 (g) → CH3CH2OO (g, gauche) + CH2CH (g) | ΔrH°(0 K) = 10.64 ± 0.90 kcal/mol | Ruscic G3X | | 0.098 | 4556.2 | CH2CHOO (g, anti) + CH3CH2 (g) → CH3CH2OO (g, gauche) + CH2CH (g) | ΔrH°(0 K) = 10.70 ± 0.90 kcal/mol | Ruscic G4 | | 0.097 | 2507.5 | [CH3C]+ (g) + CH3 (g) → [CH]+ (g) + CH3CH2 (g) | ΔrH°(0 K) = 65.21 ± 0.9 kcal/mol | Ruscic W1RO | | 0.097 | 2402.15 | CH3CH2 (g) → [CH3CH2]+ (g) | ΔrH°(0 K) = 8.124 ± 0.010 eV | Lau 2005 | | 0.096 | 2505.5 | [CH3C]- (g) + CH3 (g) → [CH]- (g) + CH3CH2 (g) | ΔrH°(0 K) = 0.13 ± 0.9 kcal/mol | Ruscic W1RO | | 0.095 | 3837.5 | (CH3)2CCH2OH (g) + CH3CH2 (g) → (CH3)3C (g) + CH2CH2OH (g, gauche-syn) | ΔrH°(0 K) = 0.57 ± 0.9 kcal/mol | Ruscic W1RO | | 0.090 | 2412.1 | CH3CH2 (g) → H (g) + CH2CH2 (g) | ΔrH°(675 K) = 153.82 ± 0.60 kJ/mol | Blitz 2021, Lightfoot 1987, Hanning-Lee 1993, Brouard 1986, Kurylo 1970, Michael 1973, Barker 1969, Barker 1970, Feng 1993, 2nd Law |
|
|
|
References
|
|
1
|
|
B. Ruscic, R. E. Pinzon, M. L. Morton, G. von Laszewski, S. Bittner, S. G. Nijsure, K. A. Amin, M. Minkoff, and A. F. Wagner,
Introduction to Active Thermochemical Tables: Several "Key" Enthalpies of Formation Revisited.
J. Phys. Chem. A 108, 9979-9997 (2004)
[DOI: 10.1021/jp047912y]
|
|
2
|
|
B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner,
Active Thermochemical Tables: Thermochemistry for the 21st Century.
J. Phys. Conf. Ser. 16, 561-570 (2005)
[DOI: 10.1088/1742-6596/16/1/078]
|
|
3
|
|
B. Ruscic and D. H. Bross,
Active Thermochemical Tables (ATcT) values based on ver. 1.148 of the Thermochemical Network (2023); available at ATcT.anl.gov |
|
4
|
|
T. L. Nguyen, J. Peeters, J.-F. Müller, A. Perera, D. H. Bross, B. Ruscic, and J. F. Stanton,
Methanediol from Cloud-Processed Formaldehyde is Only a Minor Source of Atmospheric Formic Acid
Natl. Acad. Sci. 120, e2304650120/1-8 (2023)
[DOI: 10.1073/pnas.2304650120]
|
|
5
|
|
B. Ruscic,
Uncertainty Quantification in Thermochemistry, Benchmarking Electronic Structure Computations, and Active Thermochemical Tables.
Int. J. Quantum Chem. 114, 1097-1101 (2014)
[DOI: 10.1002/qua.24605]
|
|
6
|
|
B. Ruscic and D. H. Bross,
Thermochemistry
Computer Aided Chem. Eng. 45, 3-114 (2019)
[DOI: 10.1016/B978-0-444-64087-1.00001-2]
|
|
|
|
Formula
|
|
The aggregate state is given in parentheses following the formula, such as: g - gas-phase, cr - crystal, l - liquid, etc.
|
|
|
Uncertainties
|
The listed uncertainties correspond to estimated 95% confidence limits, as customary in thermochemistry (see, for example, Ruscic [5] and Ruscic and Bross[6]).
Note that an uncertainty of ± 0.000 kJ/mol indicates that the estimated uncertainty is < ± 0.0005 kJ/mol.
|
|
|
Website Functionality Credits
|
The reorganization of the website was developed and implemented by David H. Bross (ANL).
The find function is based on the complete Species Dictionary entries for the appropriate version of the ATcT TN.
The molecule images are rendered by Indigo-depict.
The XYZ renderings are based on Jmol: an open-source Java viewer for chemical structures in 3D. http://www.jmol.org/.
|
|
|
Acknowledgement
|
|
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. DE-AC02-06CH11357.
|
![C[CH2]](../images/45.png)
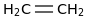
![C=[CH2+]](../images/1101.png)


![[CH2+]1[CH2][H]1](../images/106.png)

![CC=[CH2+]](../images/1545.png)
![[C]](../images/20.png)
![[C]](../images/1357.png)
![[C]](../images/2614.png)